Full Length Research Article - (2023) Volume 18, Issue 4
*Correspondence: Walid Saber Hussain, Assistant Lecturer, Faculty of Physical Therapy, 6 October University, Giza, Egypt, Email:
2Professor of Physical Therapy for Pediatrics, Faculty of Physical Therapy, Cairo University, Giza, Egypt
3Dean of Faculty of Physical Therapy, Badr University in Assiut (BUA), Egypt
4Professor and Head of Department of Physical Therapy for Pediatrics, Faculty of Physical Therapy, Cairo University, Giza, Egypt
Received: 26-Jul-2023 Accepted: 20-Aug-2023 Published: 20-Aug-2023
Abstract
Background: Children with Down syndrome (DS) are increased probability of suffer from feeding difficulties, which can have long-term health effects, such as growth deficits and lower scholastic and cognitive performance that causes a negative impact on children’s well-being.
Purposes: This study aimed to investigate the effect of neuromuscular electrical stimulation on oral motor skills and swallowing and feeding functions in children with Down syndrome.
Methods: Forty- eight children with DS from both sexes contributed to this research, with ages ranging from 3-6 years. They were randomly divided into two groups of equal numbers. The control group received an especially designed physical therapy program and oral motor training program. The study group received neuromuscular electrical stimulation (NMES) in addition to a specially designed physical therapy program and oral motor training program given to the control group. Both groups received the treatment program 3 times / week for 3 successive months. Assessment of oral motor skills was conducted by oral motor assessment scale (OMAS) and the pediatric eating assessment tool (Pedi- EAT) was used for assessment of swallowing and feeding functions. Assessment was performed before and after 3 months of intervention.
Results: The results of the present study showed statistically significant improvement within both groups in all measured variables when comparing their pre- and post-treatment mean values. Statistically significant differences were observed in all measured variables between the two groups in favor of the study group.
Conclusion: Neuromuscular electrical stimulation in conjunction with oral motor exercises is more effective than using oral motor exercises alone in improving oral motor skills and swallowing function in children with Down syndrome.
Keywords
Down syndrome, Dysphagia, Oral motor exercises, Neuromuscular electrical stimulation
Abstracto:
"Antecedentes: Los niños con síndrome de Down (SD) tienen una mayor probabilidad de sufrir dificultades de alimentación, que pueden tener efectos a largo plazo en la salud, como déficits de crecimiento y un menor rendimiento escolar y cognitivo que causa un impacto negativo en el bienestar de los niños. Propósitos: Este estudio tuvo como objetivo investigar el efecto de la estimulación eléctrica neuromuscular en las habilidades motoras orales y las funciones de deglución y alimentación en niños con síndrome de Down. Métodos: Cuarenta y ocho niños con SD de ambos sexos contribuyeron a esta investigación, con edades comprendidas entre los 3 y los 6 años. Se dividieron aleatoriamente en dos grupos de igual número. El grupo de control recibió un programa de terapia física y un programa de entrenamiento motor oral especialmente diseñados. El grupo de estudio recibió estimulación eléctrica neuromuscular (NMES) además de un programa de terapia física especialmente diseñado y un programa de entrenamiento motor oral administrado al grupo de control. Ambos grupos recibieron el programa de tratamiento 3 veces por semana durante 3 meses sucesivos. La evaluación de las habilidades motoras orales se realizó mediante la escala de evaluación motora oral (OMAS) y se utilizó la herramienta de evaluación de alimentación pediátrica (Pedi - EAT) para la evaluación de las funciones de deglución y alimentación. La evaluación se realizó antes y después de 3 meses de intervención. Resultados: Los resultados del presente estudio mostraron una mejoría estadísticamente significativa dentro de ambos grupos en todas las variables medidas al comparar sus valores medios previos y posteriores al tratamiento. Se observaron diferencias estadísticamente significativas en todas las variables medidas entre los dos grupos a favor del grupo de estudio. Conclusión: La estimulación eléctrica neuromuscular en combinación con ejercicios motores orales es más efectiva que el uso de ejercicios motores orales solos para mejorar las habilidades motoras orales y la función de deglución en niños con síndrome de Down."
Introduction
Trisomy 21, or Down syndrome (DS), is an abnormality in the genome that arises due to the existence of an extra copy of the 21st chromosome. Common symptoms include delayed physical development, cognitive limitations ranging from mild to severe, and atypical features of the face [1].
Swallowing process has are four phases including; oral preparation, oral phase, pharyngeal phase, and esophageal phase. The differentiation between stages is determined by the location of the bolus. Furthermore, each phase has a specific purpose, and it's critical to understand what constitutes a proper swallow [2].
Dysphagia is a medical disorder that impairs one's ability to consume food and liquids in a manner that is both safe and efficient. Patients with dysphagia are more prone to restrict their nutritional intake, which can lead to malnutrition and dehydration as a result [3].
There are several factors that can contribute to dysphagia in individuals with DS. One of the main reasons is the anatomical differences in the structure of the oral cavity, pharynx, and larynx. These differences can affect the coordination of the swallowing process and lead to difficulties in chewing, initiating a swallow, or coordinating the different stages of swallowing. Some DS children have sensory processing issues that affect their ability to tolerate certain textures, temperatures, or flavors of food [4].
Individuals with various genetic abnormalities typically experience difficulties swallowing due to the interplay between medical, anatomical, physiological, and behavioral aspects. Eating issues are a well-documented symptom of many genetic conditions, and Down syndrome is among them [5].
Oral Motor Exercises were designed to improve speech articulatory precision. Subsequently, these exercises were logically broadened and translated to swallowing rehabilitation to improve oral-phase motions and facilitate tonguedriving forces involved in the pharyngeal phase of swallowing [6]. Oral motor activities enhance the balance, power, and movement of the mouth's muscles to promote feeding, oral discrimination, or the perception of sensation [7].
Neuromuscular electrical stimulation is regarded as a therapy option for neurological rehabilitation that gives activation to the musculature using a surface electrode. It is utilized in enabling techniques to promote power in the muscles and sensory integration to avoid muscle wasting, therefore increasing motor performance. It is most used to elicit voluntary muscular contractions in the neck area, where larger and more motor units are recruited. Since dysphagia may be improved by promoting the musculature associated with feeding and/or enhancing perceptual impulses, it has been believed that NMES could be beneficial [8,9].
Materials and Methods
Participants
Forty-eight children with DS, including boys and girls, were involved in the current research. They were selected from the physical therapy department of The National Institute of Neuromotor System, Giza, Egypt. They were selected according to sample size calculations using the G-power test (effect size = 1, power = 95%, and α = 0.05). The aim of this research was to investigate the influence of NMES on dysphagia in children with DS, and it ran from July 2021 to June 2022. The selection for children was based on the subsequent guidelines: Their ages varied between 3 and 6. They had scores from 4 to 7 in accordance to functional oral intake scale [10]. They had score above 3 according to Pediatric Eating Assessment Tool (PEDI-EAT-10) [11]. They had the ability to move around, but with intermittent falling. Their IQ level is not less than 50 to be able to follow instructions during testing and treatment procedures [12]. The study excluded children who presented with visual or auditory impairments, infections of the chest or unstable cardiac conditions, dental deformities, infectious skin conditions, gum problems, and cognitive impairments that could potentially hinder their capacity to keep up with instructions.
Randomization
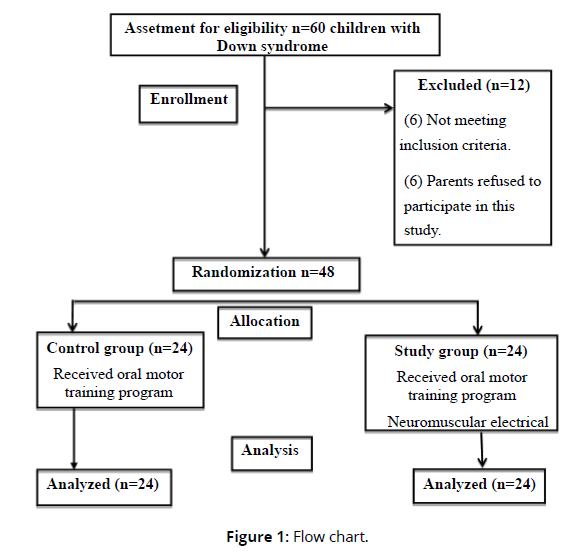
Participants in the study were divided randomly into two groups, with each group consisting of 24 children. The selection procedure was conducted using sealed envelopes. The procedure of randomization was carried out by a researcher who had no stake in the outcome of the study. Control group: included 24 children who received especially designed physical therapy program and oral motor training program. Study group: included 24 children who received neuromuscular electrical stimulation in addition to especially designed physical therapy program and oral motor training program given to the control group. Figure 1 demonstrates the study's method of recruiting and the flowchart of its experimental setup (Figure 1).
Study design
This randomized controlled trial was approved by Research Ethical Committee of the Faculty of Physical Therapy, Cairo University (no. P.T.REC/012/ 003025) and registered at Clinical Trials.gov Identifier: NCT05277142 as well as predetermined hospitals-based agreement was obtained before preceding the procedures of the study. Before starting the research project, researchers informed both the children and their parents about its goals and procedures. All the caretakers of those participating gave their informed consent.
Outcome measures
Each child was assessed at the beginning of treatment (baseline) and again after 3 months of therapy (post-treatment). Assessment of oral motor skills was conducted by oral motor assessment scale (OMAS) and the pediatric eating assessment tool (Pedi- EAT) was used for assessment of swallowing and feeding functions.
Procedures for selection
Assessment of the level of oral intake:
Functional oral intake scale (FOIS) was used to assess the level of oral intake. The mother of participating children were asked about the types of foods that the child is able to consume and the texture and consistency of the food, specifically whether it was solid, soft, or mashed [13]. After the question was posed to the mother, oral intake levels between the fourth and seventh levels were selected for this study.
Assessment of the swallowing function:
The Pediatric Version of the Eating Assessment Tool, also known as PEDIEAT- 10, was used to assess the swallowing function. It is also a valid, reliable, easy, and fast evaluation tool for children with dysphagia that providers report. Parents completed the PEDI-EAT10. Which has ten items, and each item was scored between 0 and 4. The score "0" denotes "no problem," while the score "4" denotes "severe problem." which is a 10 item each item defines a problem with the swallowing function. Higher PEDI-EAT10 scores indicate a greater risk of dysphagia and swallowing dysfunction [14,11].
Procedures for evaluation
Assessment of Oro-motor skills:
The oral-motor skills of the children in both groups were analyzed with the use of the Oral Motor Assessment Scale (OMAS). The caregiver has been told to provide the child with a regular diet of either soft, spoonable foods or solid, non-liquid foods (served in a cup, with or without straw). Children's scores on items including mastication, suction, and deglutition were determined based on the examiner's observations [15,16]. Passive = 0, sub-functional = 1, semifunctional = 2, and fully functioning = 3. (functional). Mouth closure, lip closure on the utensil, lip closure during deglutition, food control while swallowing, mastication, straw sucking, and liquid control during deglutition are the seven components of OMAS.
Assessment of swallowing and feeding functions
The Pedi EAT was used to assess swallowing and feeding functions. The instrument in question is a parent-reported survey consisting of 78 items. Its purpose is to assess symptoms related to problematic feeding in children from 6 months to 7 years who start eating some solid foods into their diet. The Pedi EAT is divided into four subscales: Physiologic Symptoms, Problematic Mealtime Behaviors, Selective/Restrictive Eating, and Oral Processing [17,18].
The Pedi-EAT items are scored based on a scale where lower scores represent fewer symptoms and higher values represent greater symptoms related to problematic feeding. The items are accompanied by numerical values denoting the corresponding score that each response would attain. The summation of scores for each item within every area was performed. At the conclusion of each section, a designated space is available for the purpose of documenting the cumulative score of said section. The manual pages' age-specific reference values were utilized to ascertain the degree of apprehension linked to the child's obtained score.
Procedures for treatment
Physical therapy program
Both groups received specially designed physical therapy program 3 times / week for a period of 3 successive months. Each session was conducted for 60 minutes for both groups.
According to Ruiz‐González and Maïano [19,20]. children in both groups received the following program:
• Enhancing posture responses from forward, backward, and sideways postures by facilitating righting and balance responses
• Balance exercises from various situations including kneeling, half kneeling and standing position by using balance board.
• Standing on one limb.
• Stoops and recovery from sitting on roll then from standing.
• Standing kicks ball with right foot then left one.
• Walking forward carrying a large ball with 2 hands.
• Standing throwing ball.
• Facilitation of protective reactions from standing position in order to help him or her take precautions against falling by moving forward, backward, or sideways.
Oral motor training program
Both groups received oral motor training for 40 minutes 3 times/week for 3 successive months: The child was positioned sitting on a chair that fit his/her body. Feet rested flat on the ground to stabilize the whole body. Knees were bent at 90 degrees over the front of the chair. Oral motor training program included the following exercises (1) Sensory Preparation Activities – ‘Wake ups’ (e.g. tap cheeks to improve sensory awareness and Cheeks and lips were massaged gently with an electric toothbrush) [21,22]. (2) Lip exercises including a-Lip closure (e.g. Put the piece of make-up sponge between child lips and hold it there and Transition to tongue depressor) [23]. b- Straw drinking (e.g. Using jumbo straw then Using regular straw with lip block) [24]. (3) Jaw exercises (e.g. Place the tips of bite blocks on the lower back molars on both sides of the mouth, extending from the front and pull forward using isometric resistance while maintaining the bite posture for 15 seconds) [25]. (4) Tongue exercises that include resistance for tongue protrusion., (e.g., Instruct the child to open his/her mouth and push firmly on the tongue tip with a tongue depressor until the blade is retracted into the oral cavity and then release) [26].
Neuromuscular electrical stimulation
Children in the study group received NMES for supra and infra hyoid muscles for 3 months, 3 sessions/ week for 20 minutes. The intensity ranged from 3 to 5mA, the frequency increased to 80 HZ,The time on and off ratio was 1:1 and the pulse width will be 500 micros. [27].
The child was positioned sitting on a chair. The anatomical position of supra and infra hyoid muscles was located as suprahyoid above hyoid bone in the neck and infrahyoid between the hyoid bone and thyroid notch [28]. Two sets of electrodes were connected to the muscles (on both sides) and the intensity increased slowly till visible or even a flicker contraction appeared (beyond contraction it increased according to the patient’s tolerance and comfort level). [29].
Statistical analysis
The data was analysed using SPSS for Windows, version 25 (a statistical programme for social scientists). All measured variables and demographic parameters such as age, weight, height, and body mass index were given descriptive statistics such as mean and standard deviation. Within-group comparisons were made using inferential statistics, such as the paired t-test and the Wilcoxon signed-ranks test, before and after the intervention. Between-group comparisons were conducted using the Mann-Whitney U test and the independent t-test. In this study, we used statistical significance level at 0.05.
Results
At baseline, Comparing the general features of the participants in both groups demonstrated that the results showed no significant difference between the two groups in the mean values of age, weight, height and BMI, FIOS and PEDIEAT- 10 (P >0.05) (Table 1).
| Control group | Study group | p-value | |
|---|---|---|---|
| X̅±SD | X̅±SD | ||
| Age (years) | 4.44 ± 0.92 | 4.59 ± 0.89 | 0.55 |
| Weight (kg) | 13.6 ± 2.87 | 13.56 ± 2.66 | 0.95 |
| Height (m) | 0.95 ± 0.11 | 0.94 ± 0.09 | 0.98 |
| BMI (kg/m²) | 15.2 ± 2.26 | 14.92 ± 1.74 | 0.62 |
| FOIS | 6.92 ± 0.28 | 6.88 ± 0.34 | 0.64 |
| PEDI-EAT-10 | 15.41 ± 1.21 | 15.04 ± 1.16 | 0.27 |
| X̅: Mean; SD: Standard deviation; BMI: Body mass index; FOIS: Functional Oral Intake Scale; PEDI-EAT-10: Pediatric Eating Assessment Tool ; p-values: Probability values. | |||
Results from both the control and study groups showed statistically significant improvements in OMAS and Pedi-Eat, post-treatment mean values compared to pre-treatment mean values (P< 0.05) (Tables 2-5).
| OMAS | Pre treatment | Post treatment | p-value | |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||
| Control group (n=24) |
Mouth closure | 1 (1-1) | 2 (2-2) | 0.001 |
| Lip closure on the utensil | 0 (1-0) | 2 (2-1) | 0.001 | |
| Lip closure during deglutition | 0 (1-0) | 2 (2-2) | 0.001 | |
| Control of the food during deglutition | 1 (1-0) | 2 (2-1) | 0.001 | |
| Mastication | 1 (1-1) | 2.5 (3-2) | 0.001 | |
| Sucking straw | 0 (1-0) | 2 (2-1) | 0.001 | |
| Control of liquids during deglutition | 1 (1-0) | 2 (2-1) | 0.001 | |
| Total score | 4 (5-4) | 12.5 (13-11) | 0.001 | |
| IQR: Interquartile range; p-values: Probability values | ||||
| OMAS | Pre treatment | Post treatment | p-value | |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||
| Study group (n=24) |
Mouth closure | 1 (1-0.25) | 3 (3-2) | 0.001 |
| Lip closure on the utensil | 0 (1-0) | 2.5 (3-2) | 0.001 | |
| Lip closure during deglutition | 0.5 (1-0) | 3 (3-2) | 0.001 | |
| Control of the food during deglutition | 1 (1-0) | 2.5 (3-2) | 0.001 | |
| Mastication | 1 (1-1) | 3 (3-3) | 0.001 | |
| Sucking straw | 0 (1-0) | 2.5 (3-2) | 0.001 | |
| Control of liquids during deglutition | 1 (1-0) | 2.5 (3-2) | 0.001 | |
| Total score | 4 (5-3) | 18 (19-17.25) | 0.001 | |
| IQR: Interquartile range; p-values: Probability values | ||||
| Pedi EAT | Pre treatment | Post treatment | p-value | ||
|---|---|---|---|---|---|
| X̅±SD | X̅±SD | ||||
| Control group (n=24) |
Physiologic symptoms | 100.75 ± 2.87 | 18 ± 2.57 | 0.001 | |
| Problematic mealtime behaviors | 88.33 ± 6.42 | 51 ± 8.06 | 0.001 | ||
| Selective/restrictive eating | 54.5 ± 2.12 | 22.67 ± 1.81 | 0.001 | ||
| Oral processing | 50.71 ± 2.59 | 25.79 ± 2.6 | 0.001 | ||
| Total Pedi EAT | 294.29 ± 10.54 | 117.46 ± 9.76 | 0.0001 | ||
| X̅: Mean; SD: Standard deviation; p-value: Probability value | |||||
| Pedi EAT | Pre treatment | Post treatment | p-value | ||
|---|---|---|---|---|---|
| X̅±SD | X̅±SD | ||||
| Study group (n=24) |
Physiologic symptoms | 100.29 ± 5.81 | 16.08 ± 3.27 | 0.001 | |
| Problematic mealtime behaviors | 87.45 ± 6.04 | 34.21 ± 4.68 | 0.001 | ||
| Selective/restrictive eating | 54.87 ± 3.51 | 20.25 ± 1.94 | 0.001 | ||
| Oral processing | 50.54 ± 2.34 | 20 ± 2.06 | 0.001 | ||
| Total Pedi EAT | 293.16 ± 11.98 | 90.54 ± 8.91 | 0.001 | ||
| X̅: Mean; SD: Standard deviation; p-value: Probability value | |||||
There was also a statistically significant difference (P< 0.05) in favour of the study group when comparing their post-treatment mean values to those of the control group (Tables 6 & 7).
OMAS |
Control group (n=15) |
Study group (n=15) |
p-value |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Mouth closure | 2 (2-2) | 3 (3-2) | 0.001 |
| Lip closure on the utensil | 2 (2-1) | 2.5 (3-2) | 0.001 |
| Lip closure during deglutition | 2 (2-2) | 3 (3-2) | 0.001 |
| Control of the food during deglutition | 2 (2-1) | 2.5 (3-2) | 0.001 |
| Mastication | 2.5 (3-2) | 3 (3-3) | 0.001 |
| Sucking straw | 2 (2-1) | 2.5 (3-2) | 0.001 |
| Control of liquids during deglutition | 2 (2-1) | 2.5 (3-2) | 0.001 |
| Total score | 12.5 (13-11) | 18 (19-17.25) | 0.001 |
| IQR: Interquartile range; p-values: Probability values | |||
Pedi EAT |
Control group (n=15) |
Study group (n=15) |
p-value |
|---|---|---|---|
| X̅±SD | X̅±SD | ||
| Physiologic symptoms | 18 ± 2.57 | 16.08 ± 3.27 | 0.02 |
| Problematic mealtime behaviors | 51 ± 8.06 | 34.21 ± 4.68 | 0.001 |
| Selective/restrictive eating | 22.67 ± 1.81 | 20.25 ± 1.94 | 0.001 |
| Oral processing | 25.79 ± 2.6 | 20 ± 2.06 | 0.001 |
| Total Pedi EAT | 117.46 ± 9.76 | 90.54 ± 8.91 | 0.001 |
| X̅: Mean; SD: Standard deviation; p-value: Probability value | |||
Discussion
Children with Down syndrome are at a higher risk of experiencing feeding difficulties and swallowing problems due to several factors, including low muscle tone, smaller oral cavity, and reduced sensory processing. These issues can lead to difficulty chewing, swallowing, or coordinating breathing while eating, which can result in aspiration or choking [30,31].
Clinically, the importance of assessment of swallowing function is to provide an indication of the child’s ability to achieve good feeding. This can be done by observing the patient's ability to chew, manipulate food, swallow saliva,and drink liquids. This approach can help identify signs of dysphagia, such as coughing, choking, gagging, drooling, and refusing to eat [32].
There are several methods that can be used to improve swallowing function in children. The most appropriate method will be depending on the explanations behind the cause of the swallowing problem, the severity of the problem, and the age and overall health of the child. Some possible methods include dietary modifications, postural adjustments, and oral motor exercises [33]. The purpose of this study was to investigate the effect of neuromuscular electrical stimulation on dysphagia in children with Down syndrome. For this purpose, Forty- eight children with DS having dysphagia participated in this study. The selection of the age of the participated children ranged from 3 to 6 years coincided with the findings of Nordstrøm et al. [34] who reported that the age at which dysphagia becomes an issue for individuals with DS is most common between the ages of 2-7 years old. During this time, children are still developing their motor skills and learning how to swallow correctly; this makes them more prone to developing dysphagia than older individuals who may already have mastered these skills.
The results of the present study showed significant changes in both groups in all measured variables including oro-motor skills and swallowing functions but with more significant improvements in favor of the study group.
The current study proved that children in both groups showed improvement in oro-motor abilities and swallowing function after intervention, which could be due to the positive effects of the selected oro-motor program. This comes in agreement with Acar et al. [35] and Seiverling et al. [36] who reported that oral motor exercises have long been used as a form of treatment for children with dysphagia, or difficulty swallowing. These exercises can be beneficial in improving the patient's swallow coordination, strength, and endurance. These types of activities may also improve oral facial muscles which is an important factor in speech language production skills and nutritional status improvement due to increased deglutition efficiency. Exercise also helps enhance sensory integration needed for feeding tasks such as drinking from a cup without spilling it.
The results of this study could be explained by the findings of Wang et al. [37] and Zapata-Soria et al. [38] who reported that oral motor exercises have numerous beneficial effects for children with dysphagia. These advantages include increased control and strength in muscles used during swallowing as well as improved coordination and sequence of tongue/lip movements when eating or drinking. Oral motor therapy has also been found to help alleviate potential pain associated with feeding which may cause a child distress during meals times. In addition, these therapies support better regulation abilities relating to saliva production among other factors.
On the contrary, Kollia et al. [39], Willging et al. [40] and Dos Santos et al. [41] reported that oral motor exercises are a common therapy used to help with pediatric dysphagia, but there are some significant disadvantages associated with this type of treatment. These include: The effects may not be long lasting due to the dynamic nature of swallowing movements and the inability for most children to understand how to properly execute specific oral motor maneuvers. Oral muscles can become over-strengthened which leads to increased jaw strain and discomfort during mealtimes. If done incorrectly or too frequently, oral motor exercises do more harm than good by reinforcing undesired patterns and responses that make it difficult for children learn new response patterns.
The post-treatment significant differences between both groups come in agreement with the outcomes of Smith et al. [42] and Matos et al.[43] as they found that patients who received NME have reported that they felt better faster than those receiving just exercise-based therapy. They suggested quicker recovery time compared to traditional treatments like oromotor therapy because it requires fewer visits with specialist therapists than traditional approaches like exercises alone or in combination with behavioral interventions.
Also, the post-treatment differences between both groups in favor of the study group could be attributed to the effect of NMES on controlling pediatric dysphagia as previous studies have shown that it can improve muscle strength in patients by stimulating neural pathways remotely through electrodes placed on the skin surface near targeted muscles [44,45].
The post treatment improvements in the study group could be explained by the work of Speyer et al. [46] and Bekteshi et al. [47] who declared that NMES is becoming increasingly popular due to its ability to improve swallowing function and reduce difficulty with feeding. The benefits of NMES for these patients include the following: helps restore normal muscle activity associated with swallowing. It can help strengthen or retrain weakened muscles, helping them better coordinate and produce proper reflexes during eating activities. It improves quality of life by allowing children to feed normally without needing assistance from family members or healthcare providers.
Also, Propp et al. [48] and Assoratgoon et al. [49] defined that NMES is being increasingly used to treat pediatric dysphagia, and reduce risk of aspiration. It can help improve muscle strength and coordination which can lead to improved safety when eating and drinking. This reduces the risk of food entering the lungs instead of going down the throat, which is known as aspiration and improved quality of life for children experiencing dysphagia. NMES has been found to significantly reduce symptoms such as coughing and choking while eating and drinking, leading to an improved quality of life for these patients.
The post-treatment significant differences between both groups could be explained by the outcomes of Dellenbach et al. [50] and Oh et al. [51] who reported that NMES can assist in enhancing power and coordination of the hyoid muscles, which can improve the patient’s ability to swallow safely and effectively. It may enhance the recruitment and activation of motor units in targeted muscles, and improve neuromuscular control. It may also facilitate cortical plasticity and reorganization of neural pathways in the brain, leading to improved motor function.
On the contrary, Balcerak et al. [52] and Barikroo & Clark [53] reported that NMES is often used to help treat pediatric dysphagia, but it does come with some potential risks that include; painful or unpleasant sensations as it may cause a sensation of pain or discomfort as the electrodes are placed directly on the skin. Risk of overstimulation may occur leading to swelling and discomfort in those areas receiving therapy.
Study limitations
The limitations of this study include difficulty in the application of NMES in some children resulting from difficulty to maintain the optimal position of electrodes. Conducting the study during Covid-19 pandemic was more difficult than the usual circumstances as precautions and cost were required for optimal hygiene.
Conclusion
It was concluded that, NMES in conjunction with oral motor exercises may be useful to improve oral motor skills and swallowing functions in Down syndrome children with dysphagia.
Acknowledgement
The authors would like to extend their deepest appreciation to the boys and girls who participated in this study and their families.
Disclosure statement
"No author has any financial interest or received any financial benefit from this research. "
Conflict of interest
"Authors report no conflict of interest. "
References
Cañizares-Prado S, Molina-López J, Moya MT, Planells E. Oral function and eating habit problems in people with down syndrome. International Journal of Environmental Research and Public Health. 2022 Feb 24;19(5):2616.
Panara K, Ahangar ER, Padalia D. Physiology, swallowing. InStatPearls [Internet] 2021 Jul 26. StatPearls Publishing.
Moroco AE, Aaronson NL. Pediatric dysphagia. Pediatric Clinics. 2022 Apr 1;69(2):349-61.
Bull MJ, Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011 Aug;128(2):393-406.
Anil MA, Shabnam S, Narayanan S. Feeding and swallowing difficulties in children with Down syndrome. Journal of Intellectual Disability Research. 2019 Aug;63(8):992-1014.
Bowen A. Dysphagia and Aspiration. InScott-Brown's Essential Otorhinolaryngology 2022 (pp. 297-300). CRC Press.
Franciotti R, Di Maria E, D’Attilio M, Aprile G, Cosentino FG, Perrotti V. Quantitative Measurement of Swallowing Performance Using Iowa Oral Performance Instrument: A Systematic Review and Meta-Analysis. Biomedicines. 2022 Sep 18;10(9):2319.
Propp R, Gill PJ, Marcus S, Ren L, Cohen E, Friedman J, Mahant S. Neuromuscular electrical stimulation for children with dysphagia: a systematic review. BMJ open. 2022 Mar 1;12(3):e055124.
Park JS, Jung YJ, Kim MJ. Effects of neuromuscular electrical stimulation synchronized with chewing exercises on bite force and masseter muscle thickness in community-dwelling older adults in South Korea: a randomized controlled trial. International Journal of Environmental Research and Public Health. 2020 Jul;17(13):4902.
Speyer R, Cordier R, Parsons L, Denman D, Kim JH. Psychometric characteristics of non-instrumental swallowing and feeding assessments in pediatrics: a systematic review using COSMIN. Dysphagia. 2018 Feb;33:1-4.
Serel Arslan S, Kılınç HE, Yaşaroğlu ÖF, Demir N, Karaduman AA. The pediatric version of the eating assessment tool‐10 has discriminant ability to detect aspiration in children with neurological impairments. Neurogastroenterology & Motility. 2018 Nov;30(11):e13432.
Mégarbané A, Noguier F, Stora S, Manchon L, Mircher C, Bruno R, Dorison N, Pierrat F, Rethoré MO, Trentin B, Ravel A. The intellectual disability of trisomy 21: differences in gene expression in a case series of patients with lower and higher IQ. European Journal of Human Genetics. 2013 Nov;21(11):1253-9.
Yi YG, Shin HI. Psychometrics of the Functional Oral Intake Scale for children with dysphagia. Journal of pediatric gastroenterology and nutrition. 2020 Nov 1;71(5):686-91.
Squires DA, Stevens J, Johnson J, Carnevale FA, Knupp AM. The Pediatric Eating Assessment Tool-10 can be used to assess feeding skills in children with neurological impairments. Dev Med Child Neurol. 2018 Aug;60(8):829-33.
Barton C, Bickell M, Fucile S. Pediatric oral motor feeding assessments: A systematic review. Physical & occupational therapy in pediatrics. 2018 Mar 15;38(2):190-209.
Ortega A. D., Ciamponi A. L., Mendes F. M and Santos M. T., Assessment scale of the oral motor performance of children and adolescents with neurological damages. Journal of Oral Rehabilitation, 2009 Sep;36(9), 653-659.
Pados BF, Thoyre SM, Park J. Age-based norm-reference values for the Pediatric Eating Assessment Tool. Pediatric Research. 2018 Aug;84(2):233-9.
Thoyre SM, Pados BF, Park J, Estrem H, McComish C, Hodges EA. The pediatric eating assessment tool: factor structure and psychometric properties. Journal of pediatric gastroenterology and nutrition. 2018 Feb 1;66(2):299-305.
Ruiz‐González L, Lucena‐Antón D, Salazar A, Martín‐Valero R, Moral‐Munoz JA. Physical therapy in Down syndrome: systematic review and meta‐analysis. Journal of Intellectual Disability Research. 2019 Aug;63(8):1041-67.
Maïano C, Hue O, Lepage G, Morin AJ, Tracey D, Moullec G. Do exercise interventions improve balance for children and adolescents with Down syndrome? A systematic review. Physical therapy. 2019 May 1;99(5):507-18.
Rosenfeld-Johnson S. Oral placement therapy for speech clarity and feeding. Talk Tools Therapy; 2009.
Mucciolo, C. Efficacy and transfer of oral motor techniques in the remediation of speech sound disorders (Doctoral dissertation, The William Paterson University of New Jersey) 2013.
Hägg M, Anniko M. Lip muscle training in stroke patients with dysphagia. Acta oto-laryngologica. 2008 Jan 1;128(9):1027-33.
Clark HM, Shelton N. Training effects of the effortful swallow under three exercise conditions. Dysphagia. 2014 Oct;29:553-63.
Howe TH. Oromotor therapy. Pediatric Dysphagia: Challenges and Controversies. 2018:119-34.
Van den Steen L, Vanderwegen J, Guns C, Elen R, De Bodt M, Van Nuffelen G. Tongue-strengthening exercises in healthy older adults: Does exercise load matter? A randomized controlled trial. Dysphagia. 2019 Jun 15;34:315-24.
Andreoli SM, Wilson BL, Swanson C. Neuromuscular electrical stimulation improves feeding and aspiration status in medically complex children undergoing feeding therapy. International journal of pediatric otorhinolaryngology. 2019 Dec 1;127:109646.
Khan YS, Bordoni B. Anatomy, head and neck, suprahyoid muscle. InStatPearls [Internet] 2022 Jun 11. StatPearls Publishing.
Tan Z, Wei X, Tan C, Wang H, Tian S. Effect of neuromuscular electrical stimulation combined with swallowing rehabilitation training on the treatment efficacy and life quality of stroke patients with dysphagia. American Journal of Translational Research. 2022;14(2):1258.
Rogers SL, Smith B, Mengoni SE. Relationships between feeding problems, eating behaviours and parental feeding practices in children with Down syndrome: A cross‐sectional study. Journal of Applied Research in Intellectual Disabilities. 2022 Mar;35(2):596-606.
Wintergerst A, López-Morales MP. Masticatory function in children with Down syndrome. Physiology & Behavior. 2021 Jun 1;235:113390.
Lashley, K., Topp, C., & Barlow, S. Sensorimotor Integration in Children with Down Syndrome: Implications for Dysphagia Assessment and Treatment. Journal of Developmental and Behavioral Pediatrics, 2020;41(9), 720-726.
Soyer T, Arslan SS, Boybeyi Ö, Demir N, Tanyel FC. The role of oral feeding time and sham feeding on oropharyngeal swallowing functions in children with esophageal atresia. Dysphagia. 2023 Feb;38(1):247-52
Nordstrøm M, Retterstøl K, Hope S, Kolset SO. Nutritional challenges in children and adolescents with Down syndrome. The Lancet Child & Adolescent Health. 2020 Jun 1;4(6):455-64.
Acar G, Ejraei N, Turkdoğan D, Enver N, Öztürk G, Aktaş G. The Effects of Neurodevelopmental Therapy on Feeding and Swallowing Activities in Children with Cerebral Palsy. Dysphagia. 2022 Aug;37(4):800-11.
Seiverling L, Jusko E, Rodriguez J, Kuljanic A, Weaver B. Multidisciplinary Approaches to Mealtime Interventions. Behavior Analytic Approaches to Promote Enjoyable Mealtimes for Autistics/Individuals Diagnosed with Autism and their Families. 2023 Jan 10:23.
Wang T, Tai J, Hu R, Fan S, Li H, Zhu Y, Wu Y, Wu J. Effect of Tongue-Pressure Resistance Training in Poststroke Dysphagia Patients With Oral Motor Dysfunction: A Randomized Controlled Trial. American Journal of Physical Medicine & Rehabilitation. 2022 Dec 1;101(12):1134-8.
Zapata-Soria M, Cabrera-Martos I, López-López L, Ortiz-Rubio A, Granados-Santiago M, Ríos-Asín I, Valenza MC. Clinical Characteristics and Rehabilitation Strategies for the Stomatognathic System Disturbances in Patients with Stroke: A Systematic Review. International Journal of Environmental Research and Public Health. 2023 Jan;20(1):657.
Kollia B, Tsiamtsiouris J, Korik P. Oral motor treatment: Effects of therapeutic feeding on articulatory skills. Journal of prevention & intervention in the community. 2019 Jan 2;47(1):14-24.
Willging JP, Miller CK, Cohen AP. Pediatric dysphagia: Etiologies, diagnosis, and management. Plural Publishing; 2019 Dec 9.
Dos Santos KW, Hugo FN, da Cunha Rodrigues E, Stein AT, Hilgert JB. Effect of oral exercises and photobiomodulation therapy in the rehabilitation of patients with mandible fractures: randomized double-blind clinical trial. Lasers in Medical Science. 2022 Apr 1:1-9.
Smith R, Bryant L, Hemsley B. Dysphagia and quality of life, participation, and inclusion experiences and outcomes for adults and children with dysphagia: a scoping review. Perspectives of the ASHA Special Interest Groups. 2022 Feb 11;7(1):181-96.
Matos KC, de Oliveira VF, de Oliveira PL, Carvalho FA, de Mesquita MR, da Silva Queiroz CG, Marques LM, Lima DL, Carvalho FM, Braga-Neto P. Combined conventional speech therapy and functional electrical stimulation in acute stroke patients with dyphagia: a randomized controlled trial. BMC neurology. 2022 Dec;22(1):1-3.
Epperson HE, Sandage MJ. Neuromuscular development in neonates and postnatal infants: implications for neuromuscular electrical stimulation therapy for dysphagia. Journal of Speech, Language, and Hearing Research. 2019 Aug 15;62(8):2575-83.
Assoratgoon I, Shiraishi N, Tagaino R, Ogawa T, Sasaki K. Sensory neuromuscular electrical stimulation for dysphagia rehabilitation: A literature review. Journal of oral rehabilitation. 2023 Feb;50(2):157-64.
Speyer R, Sutt AL, Bergström L, Hamdy S, Heijnen BJ, Remijn L, Wilkes-Gillan S, Cordier R. Reply to Dziewas, R.; Bath, PM Endpoints in Dysphagia Trials. Comment on “Speyer et al. Neurostimulation in People with Oropharyngeal Dysphagia: A Systematic Review and Meta-Analyses of Randomised Controlled Trials—Part I: Pharyngeal and Neuromuscular Electrical Stimulation. J. Clin. Med. 2022, 11, 776”. Journal of Clinical Medicine. 2022 Jun 14;11(12):3403.
Bekteshi S, Monbaliu E, McIntyre S, Saloojee G, Hilberink SR, Tatishvili N, Dan B. Towards functional improvement of motor disorders associated with cerebral palsy. The Lancet Neurology. 2023 Jan 16.
Propp R, Gill PJ, Marcus S, Ren L, Cohen E, Friedman J, Mahant S. Neuromuscular electrical stimulation for children with dysphagia: a systematic review. BMJ open. 2022 Mar 1;12(3):e055124.
Bengisu S, Demir N, Krespi Y. Effectiveness of Conventional Dysphagia Therapy (CDT), Neuromuscular Electrical Stimulation (NMES), and Transcranial Direct Current Stimulation (tDCS) in Acute Post-Stroke Dysphagia: A Comparative Evaluation. Dysphagia. 2023 May 29:1-5.
Dellenbach, C., Jaeschke, R., & Werner, C. Neuromuscular electrical stimulation in children with feeding and swallowing disorders: A systematic review and meta-analysis. Developmental Medicine & Child Neurology, 2020; 62(5), 567-581.
Oh DH, Park JS, Kim HJ, Chang MY, Hwang NK. The effect of neuromuscular electrical stimulation with different electrode positions on swallowing in stroke patients with oropharyngeal dysphagia: A randomized trial. Journal of Back and Musculoskeletal Rehabilitation. 2020 Jan 1;33(4):637-44.
Balcerak P, Corbiere S, Zubal R, Kägi G. Post-stroke Dysphagia: Prognosis and Treatment–A Systematic Review of RCT on Interventional Treatments for Dysphagia Following Subacute Stroke. Frontiers in neurology. 2022;13.
Barikroo A, Clark AL. Effects of varying transcutaneous electrical stimulation pulse duration on swallowing kinematics in healthy adults. Dysphagia. 2022 Apr;37(2):277-85.