Full Length Research Article - (2023) Volume 18, Issue 4
*Correspondence: Sally A. Asker, Department of Physical Therapy for Paediatrics, Faculty of Physical Therapy, Cairo University, Giza, Egypt, Email:
2Dean of Physical Therapy Faculty, 6th October University, Giza, Egypt
3Department of Obstetrics and Gynaecology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Received: 07-Aug-2023 Accepted: 21-Aug-2023 Published: 21-Aug-2023
Abstract
There are serious emotional, financial, demographic, as well as medical impacts associated with infertility. Over 80 million people all over the world are affected by it. It is believed that between 10% and 15% of all couples in their reproductive years experience by reproductive problems. Assisted Reproductive Technologies (ART) were first developed nearly four decades ago to aid in reducing the burden if infertility in affected couples. Today, ART is accountable for greater than 4 percent of all live births. While most of ART-born infants are healthy, concerns about the health of ART-born infants have emerged recently. There is an elevated risk of perinatal negative outcome in newborns conceived by ART. This study was aimed to detect difference in maturity of newborns conceived from fresh as well as frozen embryo transfer in comparison with those from naturally conceived. 186 full term newborns whom mothers age ranged from 25 to 35 years old with their BMI ranged from 18.5 to 29 and they were born through in vitro fertilization/ intra cytoplasmic injection (IVF/ICSI) (frozen embryo transfer (vitrification process) and fresh embryo transfer) following 37 weeks of gestation were enrolled in this study. Newborns conceived without fertility treatments (natural conception) served as a reference group. Newborns were equally distributed into three groups: Group A: 62 Naturally conceived newborns, Group B: 62 Newborns conceived from fresh embryo transfer and Group C: 62 Newborns conceived from frozen embryo transfer through vitrification process. Newborns in the three groups were assessed for maturity by New Ballard Score that was corresponded to estimated gestational age in weeks. The Kruskal-Wallis test was used to compare the maturity of newborns born by normal conception, fresh embryo transfer, as well as frozen embryo transfer. For multiple comparisons following a significant ANOVA test, the Tukey honestly significant difference (Tukey- HSD) test was employed, and for multiple comparisons following a significant Kruskal-Wallis test, the Bonferroni post hoc test was utilized. Statistical significance was assumed at the p<0.05. When comparing the three groups, it was found that the estimated gestational age significantly lowest in group B (fresh embryo transfer) in comparison with both group C (frozen embryo transfer) as well as group A (normal conception) and it was significantly lower in group A (normal conception) compared to group C (frozen embryo transfer). There was difference in maturity has been detected in newborns conceived from fresh as well as frozen embryo transfer in comparison with naturally conceived.
Keywords
Maturity. Estimated gestational age. New Ballard Score. ART offspring
Introduction
Since the birth of the first child conceived by in vitro fertilization (IVF) in 1978, ART has continued to rise in both availability and usage. The introduction embryo cryopreservation in the beginning 1980s has occurred after developments and enhancement important laboratory technologies in ART filed (Niederberger et al., 2018).
Infertility therapies such as in IVF, ICSI, artificial insemination (AI), as well as ovarian stimulation (OI) all involve ART in order to induce pregnancy. In the growing world, it is believed that between 1.5 and 6% of newborns were conceived with the help of ART (De Geyter et al.,2018) (Goldsmith et al., 2018) (Sundaram et al., 2019) and (Pinborg, 2019).
Conventional in vitro insemination as well as ICSI, in which a single spermatozoon is inserted into the oocyte cytoplasm, are both examples of the sequence of procedures known collectively as in vitro fertilization, which involves the extracorporeal fertilizing of gametes. Embryo transfer (ET), is the process of implanting an IVF or ICSI-created embryo in the uterus at the cleavage or blastocyst stage. Following fertilization, the embryo(s) with the best possible morphology are selected and transferred directly into the uterine cavity (Fresh Embryo Transfer). Embryos that meet the criteria for transfer but aren't used in the current cycle may be frozen and used in a Frozen/Thawed Embryo Transfer (FET) in the following cycle. The frozen embryo(s) can be thawed as well as transferred if the woman is unable to conceive following the fresh transfer or if she needs another child (Zegers-Hochschild et al., 2017).
Assisted reproductive technology is associated with a higher risk of neonatal problems than those from natural conception, despite recent advances in laboratory technology as well as clinical care. (Kawwass and Badell, 2018). Still, ART-conceived infants have poor perinatal outcomes in comparison with those from naturally-conceived (Westvik-Johari et al., 2021).
Antenatally, an ultrasound performed in early pregnancy is the most reliable method for determining gestational age (Butt and Lim, 2014). Postnatally, validated scoring systems to evaluate neuromuscular and physical maturity, such as the Dubowitz neurological examination and Ballard Score, can be used to estimate a neonate’s gestational age (Dubowitz et al., 1970) (Ballard et al., 1979) (Ballard et al., 1991) and (Dubowitz et al., 2005).
Clinically, determining a newborn's gestational age (GA) is useful for classifying them as either preterm, full-term, as well as small for date infant so that they can be treated appropriately. Accurate GA knowledge helps in the early detection of life-threatening conditions in newborns, including as hypoglycemia shortly following birth, septicemia, feeding problems, as well as asphyxia. Physical as well as neuromuscular characteristics such as the Dubowitz chart, Parkins' approach, the Ballard method, and the New Ballard Score (NBS) chart can be used to estimate GA in addition to last menstrual cycle (LMP) as well as initial ultrasound (US) reports (Symington and Pinalli, 2006).
In clinical practice, determining a newborn's gestational age is crucial for identifying infants who are at increased risk of complications due to their gestational age. Preterm and full-term infants' gestational ages can be accurately estimated through clinical examination using the New Ballard Score (NBS) (Md et al., 2022). The goal of this study was to detect difference in maturity of newborns conceived from fresh as well as frozen embryo transfer in comparison with those from naturally conceived.
Subjects and methods
• Study design
Cross Sectional Case Controlled study was designed to detect the difference in maturity of newborns conceived from fresh as well as frozen embryo transfer in comparison with those from naturally conceived. All parents signed a consent form that they agreed that their newborns participated in this study after an explanation of protocol.
• Ethical considerations
The approval of the protocol of this study was done by the ethics committee of the Faculty of Physical Therapy, Cairo University on 5/1/2021, NO: P.T. REC /012/003064. The trial was registered with Clinical Trials. gov NCT 04772872.
• Enrollment
186 newborns were selected from AL Mansoura University Hospitals and private centers for obstetrics and gynecology and ICSI from February 2021 till April 2023.
• Subjects
This study included 186 newborns whom born through IVF/ICSI following 37 weeks of gestation as well as those from naturally conceived. 186 newborns were distributed into three groups; Group A: 62 Naturally conceived newborns, Group B: 62 Newborns conceived from fresh embryo transfer and Group C:
62 Newborns conceived from frozen embryo transfer through vitrification process.
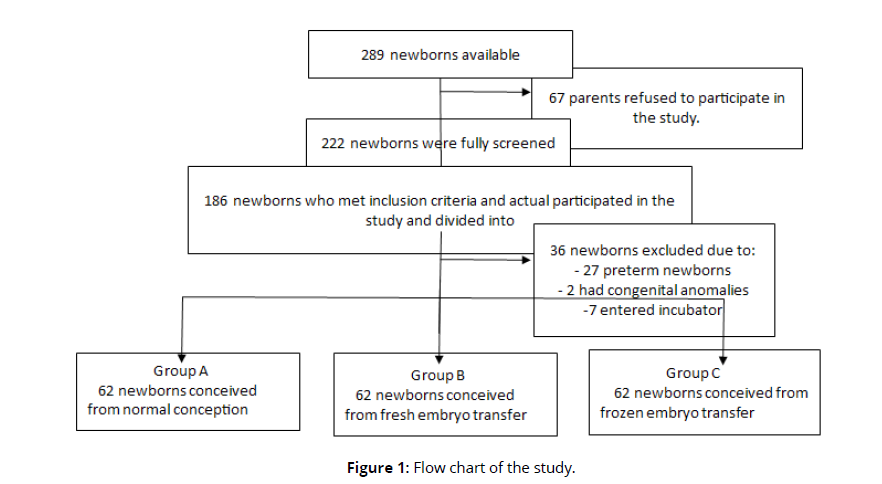
Newborn who participated in this study met the subsequent criteria: newborn’s mothers age ranged from 25 to 35 years old with their BMI ranged from 18.5 to 29 and he/she was born via IVF/ICSI (frozen embryo transfer (vitrification process) as well as fresh embryo transfer) following 37 weeks of gestation. As a reference group, newborns were born via natural conception (without any fertility treatment) were included. Exclusion criteria was the following: vanishing twins, preterm singleton, congenital abnormalities, gestational surrogates, donor oocyte pregnancy, several birth and singleton whose mother suffered from: ovarian hyper stimulation syndrome, metabolic diseases, chronic health issues, placenta previa, gestational condition, premature tear of membrane or uterine contraction, placenta accrete and cervical insufficiency and newborn whose mother was smoking. Enrolment of the newborns were shown in Figure (1) (Figure 1).
Assessment
The New Ballard Score was created by Dr. Jeanne L. Ballard (Ballard et al., 1991) as a quick and reliable means of determining the level of gestational maturity in a singleton newborn. The New Ballard score was utilized for newborns up to 4 days following delivery to assess their physical as well as neuromuscular maturity. Since the physical aspects of development progress rapidly after birth, the neuromuscular aspects showed greater stability over time. However, illness as well as medications (such as magnesium sulfate administered during delivery) altered the neuromuscular components.
For determining a newborn's clinical gestational age (GA) at birth, the New Ballard Score (NBS) is currently regarded as the most reliable approach (Erman et al., 2006). The NBS was utilized to assign gestational age whenever there was a significant difference among the obstetrically determined gestational age and the findings of physical examination; its accuracy was limited to within seven days. The maturity rating used to predict gestational age between 20 and 44 weeks of gestation. At a week of newborn´s age, the NBS provides a valid and reliable estimate of gestational age (Sasidharan et al., 2009).
As indicated in table (1), the NBS is a result of a series of steps taken to identify a newborn's gestational age via neuromuscular and physical evaluation. The maturational changes experienced by the fetus inside the uterus are utilized as a basis for the score. The neurological criteria rely heavily on measures of muscle tone, while the physical criteria are based on actual, anatomical changes. The newborn (who is less than 37 weeks old) has a natural low level of muscle tone called hypotonia. Muscle tone develops during gestation, therefore a premature newborn will have lower levels (Symington and Pinalli, 2006) (Table 1).
| 1-Neurological features | 1-Posture 2-Square window 3-Arm recoil 4-Popliteal angle 5-Scarf sign 6-Heal to ear |
| 2-External features | 1-Skin 2-Lanugo 3-Planter surface 4-Breast 5-Eye/Ear 6-Genitalia |
Scoring:
After finishing the assessment of all items of New Ballard Score, the score was given for each newborn. New Ballard Score allowed scores of each item ranged from -1 to 5, so the total scores of all items were ranged from -10 to 50. The corresponding gestational age ranged from 20 weeks to 44 weeks as shown in table (2). For scores that was among the 5-point increments, a method suggested by Ballard was extrapolated utilizing. (Ballard et al., 1991) (Table 2).
| Maturity Rating -- Ballard Method | |
| Total Score | Gestational age (Weeks) |
| -10 | 20 |
| -5 | 22 |
| 0 | 24 |
| 5 | 26 |
| 10 | 28 |
| 15 | 30 |
| 20 | 32 |
| 25 | 34 |
| 30 | 36 |
| 35 | 38 |
| 40 | 40 |
| 45 | 42 |
| 50 | 44 |
Statistical analysis
IBM's SPSS (Statistical Package for the Social Sciences) for Windows (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp) was used to analyze the data. Normality was tested utilizing of the Kolmogorov-Smirnov test. Mean and standard deviation were used to represent continuous data, whereas numbers and percentages were used to represent categorical data. Significant results were defined as those with a p-value of 0.05. Analysis of correlation between two qualitative variables using the chi-squared test. The Kruskal-Wallis test was utilized to compare the maturity of newborns born by natural conception, embryo transfer (fresh as well as frozen). Indicating which significant difference among pairs of groups was determined using the Tukey honestly significant difference (Tukey- HSD) test as a post hoc test for multiple comparisons following a significant analysis of variance (ANOVA), and using the Bonferroni post hoc test following a significant Kruskal-Wallis test. Shapiro- Wilk and Levine's tests were used to confirm that the variances among the groups were similar.
Results
Based on sample size calculation utilizing G-power test (effect size = 0.23، power = 80 %، type 1 error = 0.95 and α = 0.05). The calculated sample size was consequently 62 for each group, with an overall sample size of 186 newborns in the study. A total of 186 full-term newborns were participated in this study, all of whom were born following 37 weeks of gestation via IVF/ICSI or were naturally conceived. each group included 62 newborns. Both sexes were included. Table (3), (4) reveals a comparison of the 3 groups ´ general characteristics at baseline.
Statistical analysis for outcome measures
In terms of estimated gestational age, there was a statistically significant difference among the 3 groups (P 0.05). For group A (Normally conceived), the mean and median value of their estimated gestational age were 35.85 ± 1.88 weeks and 36.0 respectively. For group B (Fresh embryo), the mean and median value of their estimated gestational age were 35.11 ± 1.05 weeks and 35.0 respectively. For group C (Frozen embryo), the mean and median value of their estimated gestational age were 38.74 ± 0.92 weeks and 39.0 respectively.
For several comparisons following a significant ANOVA test, the Tukey honestly significant difference (Tukey- HSD) test was employed, and for multiple comparisons following a significant Kruskal-Wallis test, the Bonferroni post hoc test was utilized. The test revealed that estimated gestational age was significantly lower in group B (fresh embryo transfer) compared to group C (frozen embryo transfer) and group A (normal conception) and it was significantly lower in group A (normal conception) in comparison with group C (frozen embryo transfer) as presented in table (5) and (6). (Tables 3-6).
| Baseline characteristics Group (A) Group (B) Group (C) Normal conception Fresh embryo Frozen embryo Kruskal-Wallis (n= 62) (n= 62) (n= 62) Test value p- value |
Maternal age (years)
x̅, meanx̅ ± SD 28.80± 2.81 29.43± 2.72 29.95± 3.17 4.331 0.115* Range 25.0-35.0 25.0-35.0 25.0-35.0 BMI (kg⁄cm²) x̅ ± SD 24.30 ± 2.40 24.38 ± 2.22 24.14 ± 2.62 0.377 0.828* Range 18.9-28.4 19.9 -28.5 19.3-28.6 Paternal age (years) x̅ ± SD 32.48 ± 3.15 33.62 ± 3.81 33.73 ± 3.51 4.727 0.094* Range 28.0-39.0 26.0-42.0 25.0-42.0 Duration of pregnancy (years) x̅ ± SD 3.75± 1.85 3.31± 1.50 3.98± 1.61 4.660 0.097* Range 1.0- 7.0 1.0- 6.0 1.0- 7.0 Newborns gestational age at delivery (weeks) x̅ ± SD 38.44±0.59 38.18±0.72 38.24±0.67 4.601 0.100* Range 37.0- 39.0 37.0- 39.0 37.0- 39.0 SD, standard deviation P-value, Probability value BMI, body mass index *non-significant |
| Baseline characteristics Group (A) Group (B) Group (C) Normal conception Fresh embryo Frozen embryo ꭓ2 (n= 62) (n= 62) (n= 62) Test value p-value |
Type of labor 0.845 0.655*
ꭓ2, Chi-square testVaginal delivery 25(40.3 %) 28(45.2 %) 23(37.1 %) Cessarian section 37(59.7 %) 34(54. 8%) 39(62.9 %) Gender of singleton 4.70 0.095* Boys 35 (56.5%) 30 (48.4%) 23 (37.1 %) Girls 27 (43.5%) 32 (51.6%) 39 (62.9 %) P-value, Probability value *non-significant |
| Groups | Estimated Gestational age (weeks) x̅ ± SD |
Median | KW-value | P- value | Sig. | |
| Group A (Normally conceived) |
35.85± 1.88 | 36.0 | 108.96 | <0.001 | S | |
| Group B (Fresh embryo) |
35.11± 1.05 | 35.0 | ||||
| Group C (Frozen embryo) |
38.74± 0.92 | 39.0 | ||||
| x̅ ± SD: Mean ± Standard Deviation; KW-value: Kruskal-Wallis test; P-value: Probability value (p≤ 0.05 and 0.01 are statistically significant); S: significant. | ||||||
| Group effect | Z- Value | P-value | Sig. |
|---|---|---|---|
| Group A vs. Group B | 0.017 | 0.052 | S |
| Group A vs. Group C | 7.985 | <0.001 | S |
| Group B vs. Group C | 9.985 | <0.001 | S |
| Z-value: Mann-Whitney test; P-value: Probability value; NS: Non-significant; S: Significant at p<0.05, p<0.01 | |||
Discussion
The findings of the current study showed that there was difference in maturity of newborns conceived from fresh as well as frozen embryo transfer in comparison with those from naturally conceived. Group B (fresh embryo transfer) had a substantially smaller estimated gestational age than groups C (frozen embryo transfer) as well as A (normal conception), while group A had a substantially smaller estimated gestational age than group C (frozen embryo transfer).
However, it is still unclear what factors contribute to the higher risk of large gestational age (LGA) as well as macrosomia following frozen ET. Several theories have been made to explain this explosive growth. First, embryos of greater quality may be more likely to survive the thawing as well as freezing process, suggesting a possible selection mechanism cause. Second, cryopreservation may also change the fetus's growth capability in its earliest embryonic stages due to epigenetic modifications (heritable alterations that are not produced by changes in the structure of DNA instead through modifications, including DNA methylation). A third suggested explanation is that most frozen ET cycles do not involve the ovarian stimulation employed in a fresh IVF cycle, this could explain why the uterine environment within a frozen ET cycle is seen to be more similar to a natural uterine environment (Sine et al., 2019).
Ovarian stimulation in fresh cycles can generate supraphysiological hormone levels, which may have an effect on implantation and subsequent embryonic growth as well as development. The uterine environment in frozen ET more natural than that develops after hormonal stimulation of the ovary in fresh cycles and it's likely that the synchronisation among the endometrium as well as embryos at the time of transference is more precise in frozen cycles. These suggestions help to explain why fresh ET results in infants who are small for their gestational age, have premature births, and have low birth weights (Ming et al., 2012).
The findings provide support to the hypothesis that elevated hormone levels play a significant impact through altering the intrauterine environment. Fresh ET has been linked to a higher risk of placental anomalies than frozen ET (Healy et al., 2010; Sazonova et al., 2012; Ishihara et al., 2014; Korosec et al., 2014). In addition, IVF placentas were heavier as well as bigger than those from naturally conceived (Haavaldsen et al., 2012). Additionally, frozen ET infants had a higher perinatal mortality rate than those of fresh ET and naturally conceived (Wennerholm et al., 2013). Changes in the implantation as well as early fetal development stages may be caused to placental excessive growth, hypertensive diseases of pregnancy, and increased perinatal mortality.
There is a clear trend toward using frozen ET more frequently than fresh ET, and the frequency of frozen ET is rising quickly. Increased success rates can be attributed to advancements in cryopreservation methods, which probably result in less embryo cryodamage. This, along with growing evidence that a freeze-all policy reduces the possibility of complications during pregnancy and delivery, including premature birth, low birth weight, as well as small for gestational age. As factors that influence health as well as disease throughout one's life such as low birth weight as well as prematurity are critical to reduce in ART. (Sine and Anja, 2018).
Our results are consistent with the scientific literature, which describes an increased risk of higher birth weight and macrosomia and increased risk of LGA in infants delivered following frozen ET compared to those born following fresh ET following natural conception (NC) (Shih et al., 2008; Pelkonen et al., 2010; Henningsen et al., 2011; Feng et al., 2012; Kato et al., 2012; Nakashima et al., 2013; Roy et al., 2014; Pinborg et al., 2014; Ozgur et al., 2015 and Maas et al., 2016).
A systematic review as well as meta-analysis confirmed the findings of the study that obviously revealed that pregnancies via frozen ET have lesser relative risks of low birth weight (LBW), very low birth weight, very preterm birth, small for gestational age (SGA), placenta previa, as well as placental abruption in comparison with fresh ET (Acharya et al., 2018).
An additional meta-analysis of twenty-six studies revealed that compared to singleton pregnancies achieved through fresh ET, those achieved through frozen ET have a lower risk of premature birth, small for gestational age, as well as LBW, but a greater risk of large for gestational age (LGA) infants as well as hypertensive disorders of pregnancy (Maheshwari et al., 2018).
Compared to singleton births following either NC or fresh ET, the risk of frozen ET singleton births being LGA as well as macrosomic has been constructed by the work of Sine and Anja (2018). Frozen ET singleton infants were more likely to be born large for their gestational age (LGA) and macrosomic.
Previous investigations have found that embryo cryopreservation has been linked with a greater birth weight as well as greater incidence of LGA infants (Wikland et al., 2010; Shi et al., 2012; Belva et al., 2016; Litzky et al., 2018; Roque et al., 2019). Significant perinatal morbidity as well as elevated cardiometabolic risks are linked to LGA in infants, and these risks are often imprinted during development as well as remain throughout the child's life (Cnattingius et al., 2012) and (Chiavaroli et al., 2014).
Frozen ET is not commonly performed in a "super ovulated environment," which is defined by supraphysiologic estradiol as well as progesterone levels following ovarian stimulation (Hwang et al., 2019). Autologous fresh ET infants has been linked to be smaller compared to frozen ET infants. Estradiol levels are higher in fresh ET infants who are born small for their gestational age (Farhi et al., 2010; Imudia et al., 2012 and Royster et al., 2016).
Flavia et al., (2020) demonstrated that fresh ET is accompanied with greater rates of LBW as well as SGA, whereas frozen ET is similarly accompanied with a higher risk of LGA. A greater risk of LGA was found in children conceived via frozen ET compared to children conceived via fresh ET born from the same mother (Luke et al., 2017).
Iliodromiti et al. (2017) as well as Beta et al. (2019) provides evidence on the differences between fresh ET as well as NC and of frozen ET as well as NC, respectively. SGA, LGA, as well as preterm birth are all related to higher perinatal morbidity and mortality, in addition to infants delivered large for gestational age have a greater risk of delivery problems. Long-term negative effects are also linked to them. A higher risk of cardiovascular problems, mental health problems, as well as social challenges is connected with SGA and preterm birth, while an greater risk of obesity as well as obesity-related bad outcomes has been linked with LGA (Moster et al., 2008) and (Belbasis et al., 2016).
Westvik-Johari et al. (2021) indicated that frozen ET was accompanied with greater birthweight as well as risk of LGA, while fresh ET was accompanied with the opposite.
Although Galliano et al. observed no difference in birth weight or risk of LGA following frozen ET as well as fresh ET when comparing siblings conceived through oocyte donation but the findings of the study contradict to their conclusions (Galliano et al., 2015).
This study's findings contradict those of Cassandra et al. (2020), who suggested that fresh ET may be linked to improved implantation as well as birth outcomes in comparison with frozen ET, and that the positive impact of fresh ET was comparable across all outcomes as well as across multiple sensitivity analyses. The previous research may have used a different evaluation method and/or different inclusion criteria from the present study, which may explain why they found different findings from the present study.
Limitations
This study is limited by factors related to sample selection as lack of sufficient information regarding previous pregnancy data, causes of infertility, protocol by which endometrium was prepared, details of stimulation protocol, previous failed ICSI and difference among ART as well as non-ART parents in life style factors including sports and diet and this is due to cultural bias do not give the capacity to take all information from mother.
Conclusion
There was a significant difference in maturity of newborns conceived from fresh and frozen embryo transfer compared with those from naturally conceived.
Authors contributions
The project was developed and designed by SAA, FHA, NAZ and AW who also performed the research, and gathered, categorized, and processed the data. AW are responsible for providing sample. Research resources were provided by SAA, FHA, NAZ and AW wrote the article’s first and last drafts. The content and similarity index of the paper are the responsibility of all authors, who also critically assessed and approved the final text. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgments
The authors would like to thank all parents who agree that their newborns participated in this study.
Declaration of interest
No declarations of interest were reported by the authors.
References
Acharya, K.S., Acharya, C.R., Bishop, K., Harris, B., Raburn, D. and Muasher, S.J., 2018. Freezing of all embryos in in vitro fertilization is beneficial in high responders, but not intermediate and low responders: an analysis of 82,935 cycles from the Society for Assisted Reproductive Technology registry. Fertil Steril;110: 880–7. https://doi.org/10.1016/j.fertnstert.2018.05.024
Ballard, J., Novak, K. and Driver, M., 1979. A simplified score for assessment of fetal maturation of newly born infants. The Journal of pediatrics, 95(5 Pt 1), 769-774. https://doi.org/10.1016/S0022-3476(79)80734-9
Ballard, L., Khoury, C., Wedig, K., et al., 1991. New Ballard Score, expanded to include extremely premature infants. J Pediatr; 119 :417. https: //doi. org/ 10. 1016/s0022-3476(05)82056-6
Belbasis, L., Savvidou, M.D., Kanu, C., Evangelou, E. and Tzoulaki, I., 2016. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med; 14 (1):147. https: //doi. org/ 10.1186/s12916-016-0692-5
Belva, F., Bonduelle, M., Roelants, M., Verheyen, G. and Van Landuyt, L., 2016. Neonatal health including congenital malformation risk of 1072 children born after vitrified embryo transfer. Human Reproduction, 31 (7), P. 1610–1620. https:// doi. org/10.1093/humrep/dew103
Beta, J., Khan, N., Khalil, A., Fiolna, M., Ramadan, G., Akolekar, R., 2019. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound Obstet Gynecol; 54(3):308– 18. https:// doi. org/10.1002/uog.20279.
Butt, K. and Lim, K., 2014. Determination of gestational age by ultrasound. SOGC clinical practice guidelines. J Obstet Gynaecol Can; 36(2):171–181. https:// doi. org/10.1016/S1701-2163(15)30664-2
Cassandra, R., Rachel, L.J., Tracy, T., Nichole, E.C. and Alex, J.P., 2020. Birth outcomes are superior after transfer of fresh versus frozen embryos for donor oocyte recipients Human Reproduction ;35(12): 2850–2859. https:// doi. org/10.1093 / humrep /deaa245.
Chiavaroli, V., Marcovecchio, M.L., de Giorgis, T., Diesse, L., Chiarelli, F., et al., 2014. Progression of Cardio-Metabolic Risk Factors in Subjects Born Small and Large for Gestational Age. PLoS ONE 9(8): e104278. https:// doi. org/10.1371/ journal. pone.0104278
Cnattingius, S., Villamor, E., Lagerros, Y.T., Wikstro¨m1, A.K., and Granath, F., 2012. High birth weight and obesity---a vicious circle across generations. International Journal of Obesity;36, 1320 – 1324. https://doi.org/10.1038/ijo.2011.248
De Geyter, C., Calhaz-Jorge, C., Kupka, M.S., Wyns, C., Mocanu, E., Motrenko, T., et al., 2018. ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod;1;33(9):1586-1601. https://doi.org/10.1093/ humrep/dey242
Dubowitz, L., Dubowitz, V. and Goldberg, C. 1970. Clinical assessment of gestational age in the newborn infant. The Journal of pediatrics, 77(1), 1-10. https://doi.org/ 10.1016/s0022-3476(70)80038-5
Dubowitz, L., Ricci, D. and Mercuri, E. 2005. The Dubowtiz neurological examination of the full-term newborn. Ment Retard Dev Disabil Res Rev; 11(1):52–60. https://doi.org/10.1002/mrdd.20048.
Erman, Wayan, R. and Soetjiningsih, 2006. Clinical gestational age assessment in newborns using the new Ballard score. Paediatrica Indonesiana;46(5-6): 97-101. https:/ /doi.org/10.14238/pi46.3.2006.97-101
Farhi, J., Ben-Haroush, A., Andrawus, N., Pinkas, H., Sapir, O., Fisch, B. and Ashkenazi, J., 2010. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online; 21(3):331–337. https://doi.org/10.1016/j.rbmo.2010.04.022.
Feng, G., Zhang, B., Zhou, H., Shu, J., Gan, X., Wu, F. and Deng, X., 2012. Comparable clinical outcomes and live births after single vitrified-warmed and fresh blastocyst transfer. Reproductive Biomedicine Online, 25(5), 466–473. https://doi.org/ 10. 1016 /j.rbmo.2012.07.008
Flavia, T.S.E., Danielle, W.A., Jessica, P., Jillian, C., Mark, W., Laura, G., Graeme, S. and Maria, P.V., 2020. Neonatal outcomes in singleton pregnancies conceived by fresh or frozen embryo transfer compared to spontaneous conceptions: a systematic review and meta-analysis. Archives of Gynecology and Obstetrics 302(1):31–45. https:// doi.org / 10.1007/s00404-020-05593-4
Galliano, D., Garrido, N., Serra-Serra, V., and Pellicer, A., 2015. Difference in birth weight of consecutive sibling singletons is not found in oocyte donation when comparing fresh versus frozen embryo replacements. Fertility and Sterility, 104(6), 1411–1418. https:// doi.org / 10.1016/j.fertnstert.2015.08.013
Geyter, C., 2019. Assisted reproductive technology: impact on society and need for surveillance. Best Pract Res Clin Endocrinol Metab;33(1):3–8. https:// doi.org / 10. 1016/j.beem.2019.01.004.
Goldsmith, S., McIntyre, S., Badawi, N. and Hansen, M., 2018. Cerebral palsy after assisted reproductive technology: a cohort study. Dev Med Child Neurol;60(1):73–80. https:// doi.org / 10.1111/dmcn.13577.
Haavaldsen, C., Tanbo, T. and Eskild, A., 2012. Placental weight in singleton pregnancies with and without assisted reproductive technology: A population study of 536,567 pregnancies. Human Reproduction, 27(2), 576–582. https:// doi.org / 10.1093/ humrep/der428.
Healy, D. L., Breheny, S., Halliday, J., Jaques, A., Rushford, D., Garrett, C., Baker, H. W. G., 2010. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Human Reproduction, 25(1), 265–274. https://doi.org/10.1093/humrep/dep376
Henningsen, A.K., Pinborg, A., Lidegaard, O., Vestergaard, C., Forman, J.L. and Andersen, A.N. 2011. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertility and Sterility, 95(3), 959–963. https://doi.org/10.1016/ j.fertnstert .2010.07.1075
Hwang, S.S., Dukhovny, D., Gopal, D., Cabral, H., Diop, H., Coddington, C.C. and Stern, J.E., 2019. Health outcomes for Massachusetts infants after fresh versus frozen embryo transfer. Fertil Steril;112(5): 900–907. https://doi.org/10.1016/ j.fertnstert. 2019.07.010
Iliodromiti, S., Mackay, D.F., Smith, G.C., Pell, J.P., Sattar, N., Lawlor, D.A., et al., 2017. Customised and non-customised birth weight centiles and prediction of stillbirth and infant mortality and morbidity: a cohort study of 979,912 term singleton pregnancies in Scotland. PLoS Med; 14(1). https://doi.org/10.1371/journal. pmed. 1002228
Imudia, A.N., Awonuga, A.O., Doyle, J.O., Kaimal, A.J., Wright, D.L., Toth, T.L. and Styer, A.K., 2012. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril ;97(6):1374–1379. https://doi.org/10.1016/j.fertnstert.2012.03.028.
Ishihara, O., Araki, R., Kuwahara, A., Itakura, A., Saito, H. and Adamson, G.D., 2014. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: An analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertility and Sterility, 101(1), 128–133. https://doi.org/10.1016/j.fertnstert. 2013. 09.025.
Kato, O., Kawasaki, N., Bodri, D., Kuroda, T., Kawachiya, S., Kato, K., and Takehara, Y., 2012. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. European Journal of Obstetrics and Gynecology and Reproductive Biology, 161(1), 46–50. https://doi.org/10.1016/j.ejogrb.2011.12.005
Kawwass, J. and Badell, M., 2018. Maternal and fetal risk associated with assisted reproductive technology. Obstet Gynecol,132(3):763–72. https://doi.org/10.1097 /AOG. 0000000000002786.
Korosec, S., Ban Frangez, H., Verdenik, I., Kladnik, U., Kotar, V., Virant-Klun, I. and Vrtacnik Bokal, E., 2014. Singleton pregnancy outcomes after in vitro fertilization with fresh or frozen-thawed embryo transfer and incidence of placenta praevia. Biomed Research International, 431797. https://doi.org/ 10.1155/2014/431797
Litzky, J.F., Sheree, L.B., Navid, E., Yujia, Z., Dmitry, M.K., Regan, N.T. and Carmen, J.M., 2018. Effect of frozen/thawed embryo transfer on birthweight, macrosomia, and low birthweight rates in US singleton infants Am J Obstet Gynecol;218(4):433.e1-433.e10. https://doi.org/ 10.1016/j.ajog.2017.12.223.
Luke, B., Brown, M.B., Wantman, E., Stern, J.E., Toner, J.P. and Coddington, C.C., 2017. Increased risk of large-for gestational age birthweight in singleton siblings conceived with in vitro fertilization in frozen versus fresh cycles. Journal of Assisted Reproduction and Genetics, 34(2), 191–200. https://doi.org/10.1007/s10815-016-0850-x.
Maas, K., Galkina, E., Thornton, K., Penzias, A. S., and Sakkas, D., 2016. No change in live birthweight of IVF singleton deliveries over an 18-year period despite significant clinical and laboratory changes. Human Reproduction, 31(9), 1987–1996. https:// doi.org/ 10.1093/humrep/dew173.
Maheshwari, A., Pandey, S., Amalraj, R.E., et al., 2018. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update;24(1):35–58. https://doi.org/ 10.1093/humupd/dmx031.
Md, A., Nazneen, N., Shahabuddin, M. and Md. F., 2022. Assessment of gestational age among Bangladesh New Born Infant by New Ballard Score. Sch J App Med Sci, 10(2): 239-244. https://doi.org/10.36347/sjams.2022.v10i02.017
Ming, L., Liu, P., Qiao, J., Lian, Y., Zheng, X., Ren, X., Wu, Y., et al., 2012. Synchronization between embryo development and endometrium is a contributing factor for rescue ICSI outcome. Reproductive BioMedicine Online, 24(5), 527–531. https://doi.org/10.1016/j.rbmo.2012.02.001
Moster, D., Lie, R.T. and Markestad, T., 2008. Long-term medical and social consequences of preterm birth. N Engl J Med; 359(3):262–73. https://doi.org/ 10.1056/ NEJMoa0706475.
Nakashima, A., Araki, R., Tani, H., Ishihara, O., Kuwahara, A., Irahara, M., Sakumoto, T., et al., 2013. Implications of assisted reproductive technologies on term singleton birth weight: An analysis of 25,777 children in the national assisted reproduction registry of Japan. Fertility and Sterility, 99(2), 450–455. https://doi.org/ 10.1016/j.fertnstert.2012.09.027
Niederberger, C., Pellicer, A., Cohen, J., Gardner, D., Palermo, G., O’Neill, C., et al. 2018. Forty years of IVF. Fertil Steril,110(2):185–324 e5. https://doi.org/ 10.1016/j.fertnstert.2018.06.005.
Ozgur, K., Berkkanoglu, M., Bulut, H., Humaidan, P. and Coetzee, K., 2015. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: Retrospective analysis. Fertility and Sterility, 104(4), 899–907. https://doi.org/10.1016/ j. fertnstert.2015.06.031
Pelkonen, S., Koivunen, R., Gissler, M., Nuojua-Huttunen, S., Suikkari, A.-M., Hyden-Granskog, C., Hartikainen, A.L., et al., 2010. Perinatal outcome of children born after frozen and fresh embryo transfer: The Finnish cohort study 1995–2006. Human Reproduction, 25(4), 914–923. https://doi.org/10.1093/humrep/dep477.
Pinborg, A., 2019. Short- and long-term outcomes in children born after assisted reproductive technology. BJOG;126(2):145–8. https://doi.org/ 10.1111/1471-0528.15437
Pinborg, A., Henningsen, A. A., Loft, A., Malchau, S. S., Forman, J. and Andersen, A. N., 2014. Large baby syndrome in singletons born after frozen embryo transfer (FET): Is it due to maternal factors or the cryotechnique? Human Reproduction (Oxford, England), 29(3), 618–627. https://doi.org/10.1093/humrep/det440.
Roque, M., Haahr, T., Geber, S., Esteves, S.C. and Humaidan, P., 2019. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update; 25(1):2–14. https://doi.org/ 10.1093/humupd/dmy033.
Roy, T. K., Bradley, C. K., Bowman, M. C., and McArthur, S. J., 2014. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertility and Sterility, 101(5), 1294–1301. https://doi.org/10.1016/j.fertnstert.2014.01.046
Royster, G.D., Krishnamoorthy, K., Csokmay, J.M., Yauger, B.J., Chason, R.J., DeCherney, A.H., Wolff, E.F. and Hill, M.J., 2016. Are intracytoplasmic sperm injection and high serum estradiol compounding risk factors for adverse obstetric outcomes in assisted reproductive technology? Fertil Steril; 106:363–370.e3. https://doi.org/10.1016/j.fertnstert.2016.04.023
Sasidharan, K., Dutta, S. and Narang, A., 2009. Validity of New Ballard Score until 7th day of postnatal life in moderately preterm neonates. Arch Child Fetal Neonatal Ed; 94(1): F39-F44. https://doi.org/10.1136/adc.2007.122564.
Sazonova, A., Kallen, K., Thurin-Kjellberg, A., Wennerholm, U. B., and Bergh, C., 2012. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Human Reproduction, 27(5), 1343–1350. https://doi.org/ 0.1093/humrep/des036
Shi, W., Xue, X., Zhang, S., Zhao, W., Liu, S., Zhou, H., Wang, M. and Shi, J., 2012. Perinatal and neonatal outcomes of 494 babies delivered from 972 vitrified embryo transfers. Fertil Steril ;97(6): 1338–1342. https://doi.org/ 10.1016/j. fertnstert. 2012. 02.051
Shih, W., Rushford, D. D., Bourne, H., Garrett, C., McBain, J. C., Healy, D. L., and Baker, H. W., 2008. Factors affecting low birthweight after assisted reproduction technology: Difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Human Reproduction, 23(7), 1644– 1653. https://doi.org/ 10.1093/humrep/den150.
Sine, B., Anja, P., 2018. Large for gestational age and macrosomia in singletons born after frozen/thawed embryo transfer (FET) in assisted reproductive technology (ART). Wiley Periodicals, Inc Birth Defects Research; 110(8):630–643. https://doi.org/ 10. 10 02/bdr2.1219
Sine, B., Viveca, S. A., Ulla-Britt, W., Hannele, L., Anne, L., Nan, B. O., Liv, B. Romundstad., Christina, B., and Anja, P., 2019. The health of children conceived by ART: ‘the chicken or the egg?’ Human Reproduction Update; 25(2):137–158. https://doi.org/ 10.1093/humupd/dmz001
Sunderam, S., Kissin, D.M., Zhang, Y., Folger, S.G., Boulet, S.L., Warner, L., et al., 2019. Assisted reproductive technology surveillance-United States, 2016. MMWR Surveill Summ;68(4):1–23. https://doi.org/ 10.15585/mmwr.ss6804a1
Symington, A. and Pinalli, J., 2006. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev,(2) 4C D001814. https://doi.org/10.1002/14651858.CD001814.pub2
Wennerholm, U.B., Henningsen, A.K. A., Romundstad, L. B., Bergh, C., Pinborg, A., Skjaerven, R.,Tiitinen, A., et al., 2013. Perinatal outcomes of children born after frozen-thawed embryo transfer: A Nordic cohort study from the CoNARTaS group. Human Reproduction, 28(9), 2545–2553. https://doi.org/10.1093/humrep/det272.
Westvik-Johari, K., Romundstad, L., Lawlor, D., Bergh, C., Gissler, M., Henningsen, K., et al., 2021. Separating parental and treatment contributions to perinatal health after fresh and frozen embryo transfer in assisted reproduction: A cohort study with within sibship analysis. PLoS Med; 25 :18(6). https://doi.org/10.1371/journal.pmed.1003683
Wikland, M., Hardarson, T., Hillensjo, T., Westin, C., Westlander, G., Wood, M. and Wennerholm, U.B., 2010. Obstetric outcomes after transfer of vitrified blastocysts. Hum Reprod ;25(7):1699–1707. https://doi.org/10.1093/humrep/deq117.
Zegers-Hochschild, F., Adamson, G., Dyer, S., Racowsky, C., de Mouzon, J., Sokol, R., et al., 2017. The international glossary on infertility and fertility care.Hum Reprod 32(9):1786–1801. https://doi.org/10.1093/humrep/dex234.