Research Article - (2024) Volume 19, Issue 1
*Correspondence: Nevein Nabil Alagamy, Lecturer, Department of Physical Therapy for Internal Medicine and Geriatric Department, Faculty of Physical Therapy, Horus University, New Damietta, Egypt, Email:
2Assistant Professor, Physiotherapy Department, Faculty of Allied Medical Sciences, Al-Ahliyya Amman University, Jordan
3Professor, Department of Physical Therapy, Imam Abdelrahman Bin Faisal University, KSA
4Lecturer, Department of Physical Therapy for Womens Health, Modern University for Technology and Information University, Cairo, Egypt
5Physician, Mansoura University Hospital, Mansoura University, Egypt
6Lecturer, Department of Physical Therapy for Pediatrics and Surgery, Modern University for Technology and Information University, Cairo, Egypt
Received: 15-Feb-2024 Published: 21-Feb-2024
Abstract
Background: The interplay between physical activity and metabolic health, particularly in diabetes management, is a pivotal area of investigation. This study examines the impact of a three-month moderate aerobic exercise regimen on glycemic control and estrogen levels in young female diabetic patients, aiming to shed light on potential synergies between exercise interventions and hormonal dynamics.
Methods: A one-group pretest-posttest design focused on young diabetic females aged 13 to 18. A structured moderate aerobic exercise program was implemented, comprising warm-up, exercise, and cool-down phases. Pre and post-intervention assessments included blood glucose, estrogen (E2) levels in the follicular phase, and fitness scores, with subsequent comparisons and correlations.
Results: Fifteen young females, with a mean age of 15.07 ± 1.49, participated in the study. The exercise program led to a reduction in glycated hemoglobin (HbA1c) from 8.19 ± 1.11% to 7.23 ± 0.94, a decrease in total daily insulin from 0.58 ± 0.04 to 0.53 ± 0.05, and an increase in estrogen levels from 54.53 ± 4.09 to 4.09. Furthermore, fitness scores improved from 66.53 ± 4.93 to 70.13 ± 4.55. Notably, a significant negative correlation between estrogen levels and glycated hemoglobin (HbA1c) levels (r = -0.813) was observed post-intervention.
Conclusion: This study demonstrates that a three-month moderate aerobic exercise regimen enhances glycemic control and estrogen levels in young diabetic females. Additionally, a robust inverse correlation between estrogen and HbA1c levels highlights a potential interplay between hormonal dynamics and metabolic outcomes in response to exercise interventions.
Keywords
aerobic exercise regimen, estrogen levels, young diabetic females.
Introduction
Type 1 diabetes mellitus (T1DM) is a medical condition of an autoimmune nature, wherein the beta cells responsible for making insulin in the pancreas are targeted and destroyed. This process leads to a lack of insulin and subsequent elevation of blood glucose levels, known as hyperglycemia [1]. The pathogenesis of this condition includes a combination of genetic and environmental variables that ultimately result in the immune-mediated destruction of beta cells and subsequent impairment of insulin production [2].
The global incidence of T1DM is rising, as evidenced by current estimates indicating that around 1.1 million individuals aged 20 years and below are affected by T1DM worldwide [3].
Insulin resistance occurs when peripheral tissues, such as adipose, skeletal muscle, and liver, exhibit inadequate responsiveness to insulin, leading to impaired glucose uptake. While insulin resistance serves as the fundamental cause of type 2 diabetes mellitus (T2DM), its exacerbation in the context of T1DM can result in compromised glycemic control [4]. Estrogen appears to exert an influence on diverse insulin-sensitive tissues and organs, contributing to the enhancement of glucose homeostasis by improving glucose-stimulated insulin secretion, improving insulin response in the liver and adipocytes, improving insulin-stimulated glucose absorption in the skeletal muscles, and improving nitric oxide production in the vascular endothelium [5].
Managing T1DM necessitates the administration of exogenous insulin to regulate and maintain appropriate blood glucose levels. The primary objective of intensive insulin therapy, whether administered through numerous daily injections or continuous subcutaneous insulin infusion in conjunction with regular blood glucose monitoring, is to maintain glycated hemoglobin (HbA1c) levels below 7% [6]. This approach mitigates the potential for enduring microvascular and macrovascular consequences [7]. The attainment of ideal glycemic control continues to provide a significant barrier, given the inherent risk of hypoglycemia among patients. In the management of T1DM, lifestyle modifications play a crucial role. These modifications encompass various aspects, including nutrition therapy, engaging in physical exercise, and adopting healthy coping skills. The primary objective of medical nutrition therapy is to effectively manage blood glucose control by coordinating carbohydrate intake and insulin activity. Regular participation in physical activity has been shown to impact glycemic management positively. Providing psychosocial support and comprehensive training in diabetic self-management skills empowers patients to control their diabetes condition effectively [8, 9].
Aerobic exercise constitutes low-intensity and prolonged physical activities reliant on oxygen consumption to generate energy in the form of adenosine triphosphate [10]. The investigation of incorporating aerobic exercise among individuals diagnosed with diabetes has yielded promising outcomes, encompassing enhanced blood glucose control, improved lipid profile, and attainment of more favorable body weight [11]. Aerobic exercise can enhance insulin sensitivity through many molecular mechanisms: The utilization of free fatty acids as a metabolic substrate leads to a decrease in the production of adipokines, reducing inflammation and enhancing insulin resistance. Additionally, it reduces oxidative stress associated with insulin resistance [12]. Moreover, it upregulated GLUT-4 in the cell membrane of insulindependent cells. Aerobic exercise has also been found to effectively decrease plasma concentrations of ceramide and mitigate the development of insulin resistance caused by ceramide. Furthermore, there has been a rise in IRS-1 phosphorylation, which subsequently enhances the insulin signal transduction process. In addition, it enhances the functionality of islets, preserves the structure of β cells, and inhibits apoptosis in β islets. Ultimately, it stimulates the process of angiogenesis in skeletal muscle, resulting in increased glucose absorption by myocytes [13, 14].
Aerobic exercise's effect on estrogen levels has also been studied among postmenopausal women and showed positive results after 12 weeks of practice, which was better than anaerobic exercise [15]. It was aimed in this study to evaluate the efficacy of aerobic exercise on fitness score, blood glucose level, and blood estrogen level in adolescent girls with T1DM.
Materials and Methods
Study design
This study was a one-group pretest–posttest design and was conducted at the internal medicine clinic of El-Hussien Hospital, Egypt, during the period from MAY 2023 to JULY 2023. The ethics committee of the Research Ethical Committee (REC) at Faculty of Physical Therapy, Modern University for Technology and Information (MTI) approved the study with an approval number of [REC/211/ MTI.PT/2303124], and clinical trial number [NCT06139913]. Written informed consent was taken from the participants after discussing the risks and benefits of the interventions.
Participants
A sample size of 10 was determined as a prerequisite for the study design to address the research question, assuming a statistical power of 90%, a type I error rate of 5%, a standard deviation of 10, and an anticipated increase in the fitness score of 10. The inclusion criteria comprised females aged 13 to 18 diagnosed with T1DM for over 3 years, undergoing treatment with an insulin dose of ≥ 0.5 IU/Kg/day, and possessing a body mass index (BMI) between 25 and 30 kg/m2. Exclusion criteria encompassed individuals with irregular menstrual cycles, those experiencing severe and frequent hypoglycemia in the last 6 months, defined as two or more episodes per week with a blood glucose level ≤ 60 mg/dl, and individuals with any medical condition that impedes aerobic exercise.
Interventions
It included applying moderate aerobic exercise as described by The American College of Sports Medicine (ACSM) [16, 17], preceded by a 10-minute warmup phase and cooling down. The warm-up regimen incorporated preliminary stretching exercises targeting the calf and hamstring muscles, lasting 5 minutes. Additionally, participants walked on a treadmill at an intensity level sufficient to elevate the heart rate (HR) by up to 10 beats per minute from the resting baseline. The moderate aerobic exercise protocol involved utilizing a treadmill for 50 minutes, administered thrice weekly over 12 weeks. This was achieved by maintaining an exercise intensity set at 65%–70% of the maximum HR, commonly called the target HR (THR). The cool-down phase comprised a resting period lasting 10 minutes, during which participants were seated on a chair and engaged in deep breathing exercises. The cool-down session persisted until the patient's pulse rate returned to the resting baseline. The exercise program was terminated prematurely in response to reported instances of chest pain, hypoglycemia symptoms, or a decrease in oxygen saturation below 95%.
Assessments and follow-up
Per protocol, the following assessment was carried out at every session, preand post-interventions: (1) Vital signs, including HR, blood pressure, and oxygen saturation. (2) Body Mass Index (BMI) was calculated as weight in kg divided by the square of the height in meters. (3) Fitness score was assessed through multifrequency bioelectrical impedance analysis. Demographic data were inputted into the apparatus, after which the subjects held palm electrodes, initiating the machine. Subsequently, the machine delivered results within a 3-5 minute timeframe. (4) THR values were calculated based on the maximum HR equal to age years subtracted from 220. (5) Blood glucose level including fasting, post-prandial, and glycated levels. (6) Estradiol blood level (E2) was analyzed at the follicular phase using micro enzyme-linked immunoassays (ELISAs) and an autoanalyzer (Abbott Laboratories, Wiesbaden, Germany).
Statistical analysis
Statistical analysis was performed using the paired samples t-test for normal distribution variables. The correlation between variables was assessed using the Pearson correlation test. Multivariate linear regression was conducted to determine the associated factors with the improvement in HbA1c after ensuring that the model assumptions were met. All statistical tests were conducted at a significance level of 0.05. The analysis used the Statistical Package for Social Science (SPSS) version 27.
Results
Patients and baseline
Included in this study were fifteen female patients aged 13 to 18 years, with a BMI ranging from 23.6 to 28.7 kg/m². Initial characteristics revealed variability in total daily insulin units (0.51 to 0.64 IU/kg/day) and glucose levels (fasting up to 298 mg/dl, post-prandial up to 313 mg/dl, and HbA1c up to 9.7%). A more detailed overview is provided in Table 1. Correlation analysis among baseline variables unveiled a positive association between HbA1c and BMI (Rho = 0.636, p = 0.011) and a negative correlation between HbA1c and fitness score (Rho = -0.514, p = 0.05). Notably, baseline estrogen levels did not exhibit a significant correlation with HbA1c (Rho = 0.192, p = 0.494).
| Pre-intervention | Post-intervention | p-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 15.07 | 1.486 | |||
| BMI pre | 26.333 | 1.5323 | |||
| Insulin unit | 0.5813 | 0.0417 | 0.526 | 0.048 | <0.001* |
| Fitness score | 66.53 | 4.926 | 70.13 | 4.549 | <0.001* |
| FBG | 257.27 | 29.502 | 194.8 | 34.6 | <0.001* |
| post prandial | 242.8 | 44.127 | 189.8 | 39.757 | <0.001* |
| HbA1c | 8.193 | 1.1094 | 7.233 | 0.942 | <0.001* |
| Estrogen (E2) | 54.53 | 4.086 | 65.67 | 5.407 | <0.001* |
Outcomes
After implementing the 3-month exercise program, notable improvements were observed in various health markers. Fasting blood glucose exhibited a substantial reduction with mean ± standard deviation (SD) of 62.47 ± 28.62 mg/ dl. Post-prandial blood glucose also decreased significantly, showing a decline of 53.00 ± 17.57. HbA1c levels demonstrated a noteworthy decrease by 0.96 ± 0.216%. Additionally, there was a significant decrease in the insulin total daily dose by 0.055 ± 0.019 IU/kg/day. Hormonal changes were observed as well, with a significant increase in estrogen levels by 11.13 ± 7.25. Furthermore, participants experienced a meaningful improvement in fitness scores, indicating a rise of 3.6 ± 0.91.
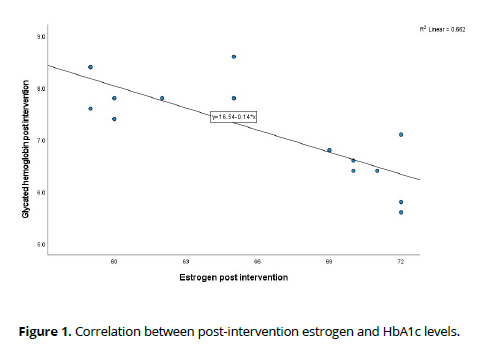
Following the intervention, correlation analysis among post-intervention variables revealed compelling associations. Notably, a substantial negative correlation emerged between estrogen levels and HbA1c (Rho = -0.813, p < 0.001), indicating a potential influence of estrogen on glycemic control. This noteworthy relationship is visually depicted in Figure 1.
Furthermore, a significant negative correlation was observed between fitness score and HbA1c (Rho = -0.528, p = 0.043), underscoring the potential impact of improved fitness on glycemic outcomes.
Factors associated with glycemic control
The results of the multivariate linear regression analysis, detailed in Table 2, illuminated the independent effects of baseline BMI, baseline fitness score, and baseline estrogen level on post-intervention HbA1c levels. Patients with References higher baseline BMI demonstrated a significant positive association with postintervention HbA1c levels (Beta = 0.324), indicating that elevated baseline BMI was independently linked to higher glycated hemoglobin levels after the intervention.
Independent variables |
Beta | SE | t-test value | p-value |
|---|---|---|---|---|
| Pre-intervention | ||||
| (Constant) | -0.542 | 4.913 | -0.11 | 0.914 |
| Age | -0.103 | 0.103 | -0.997 | 0.345 |
| BMI | 0.324 | 0.109 | 2.962 | 0.016* |
| Insulin unit | 1.21 | 3.523 | 0.343 | 0.739 |
| Fitness score | -0.106 | 0.034 | -3.161 | 0.012* |
| Estrogen | 0.132 | 0.044 | 2.981 | 0.015* |
| Post-intervention | ||||
| (Constant) | 18.225 | 3.259 | 5.592 | <0.001 |
| Age | -0.085 | 0.128 | -0.666 | 0.52 |
| Insulin unit | 2.749 | 3.401 | 0.808 | 0.438 |
| Fitness score | -0.062 | 0.038 | -1.641 | 0.132 |
| Estrogen | -0.104 | 0.039 | -2.641 | 0.025* |
Beta: Regression coefficient. BMI: Body mass index. SE: Standard error. *: Significant p-value at 0.05 level of significance
Similarly, lower baseline fitness scores were independently associated with higher post-intervention HbA1c levels (Beta = -0.106). This suggests that individuals with lower baseline fitness experienced a less favorable response in terms of glycemic control following the intervention.
Moreover, elevated baseline estrogen levels exhibited an independent positive effect on post-intervention HbA1c levels (Beta = 0.132). Patients with higher baseline estrogen levels were associated with higher HbA1c levels after the intervention.
Interestingly, lower post-intervention estrogen levels were independently linked to higher post-intervention HbA1c levels (Beta = -0.104). This implies that a reduction in estrogen levels post-intervention was associated with an unfavorable impact on glycemic control.
The detailed insights from this multivariate analysis underscore the nuanced relationships between baseline characteristics and post-intervention glycemic outcomes, providing valuable information for understanding the factors influencing HbA1c levels in response to the intervention.
Discussion
In this research, we investigated the impact of an exercise program on diabetic control and estrogen levels. Our findings revealed substantial and statistically significant effects, shedding light on the potential interplay between physical activity, diabetes management, and hormonal regulation. These results contribute valuable insights to the ongoing discourse on holistic approaches to health interventions for individuals with diabetes. The moderate aerobic exercise program for 3 months applied in this study could improve glycemic control and decrease total daily insulin dose. This improvement was associated with an improvement in the fitness score and an increase in the estrogen level.
Numerous prior studies examining aerobic exercise's impact on managing T1DM have yielded promising findings. A meta-analysis including 33 studies found that aerobic training was an effective tool for enhancing chronic glycemic control of patients with T1DM. In contrast, resistance training, mixed training approaches, and high-intensity interval exercise did not yield statistically significant improvements in chronic glycemic control [18].
These results underscore the preferential impact of aerobic training on managing glycemic levels while highlighting the limited efficacy of other training modalities in this regard. While resistance exercise initially induces a milder decline in blood glucose during activity compared to aerobic exercise, it is linked to more sustained reductions in post-exercise glycemia. This suggests a distinctive impact of resistance exercise characterized by a prolonged and beneficial influence on post-exertion blood glucose levels as opposed to the immediate effects observed in aerobic exercise [19].
Consistent with our study findings, a recent meta-analysis encompassing eighteen controlled trials, involving a total of 972 participants with T2DM, shed light on the impact of exercise on glycemic control. Within the meta-analysis, 523 participants were assigned to an exercise group, while 449 were assigned to a control group. The pooled analysis revealed compelling evidence in favor of mild to moderate intensity aerobic exercise. In the one-group comparison, aerobic exercise was associated with a significant improvement in HbA1c (Mean Difference (MD) 0.35, 95% Confidence Interval (CI) 0.23-0.48). Moreover, in the two-group comparison, the exercise group exhibited a superior improvement in HbA1c compared to the control group (MD-0.46, 95% CI-0.69 to-0.22) [20].These findings from a diverse range of studies further emphasize the consistent and positive impact of aerobic exercise on glycemic outcomes in individuals with DM.
In recognition of the necessity for a well-defined, supervised, and consistent aerobic exercise program to achieve its objectives, we instituted a comprehensive multidisciplinary program. This program featured meticulous supervision, encompassing the expertise of a physiotherapist, endocrinologist, nutritionist, sociologist, and dietitian. This collaborative approach aimed to ensure the thorough description and effective implementation of the aerobic exercise program, optimizing its potential to meet specified goals.
Aligning with our perspective, a study involving 28 participants with T1DM investigated the impact of unsupervised home-based aerobic exercise on glycemic control. Despite the three-month home-based aerobic exercise program duration, the study revealed no statistically significant effects on HbA1c [21]. These findings reinforce the need for further exploration and consideration of alternative approaches to enhance glycemic control in individuals with T1DM engaging in home-based aerobic exercise, such as a supervised exercise program by a physiotherapist.
On the other hand, few studies exploring the effect of exercise on estrogen levels found conflicting results. It was reported in a very early study that engagement in physical exercise serves as a physiological stimulus, leading to notable elevations in plasma concentrations of estradiol and progesterone. Particularly, these elevations are more pronounced during the luteal phase of the menstrual cycle. It is noteworthy that the observed increases in both estradiol and progesterone exhibit a correlation with the intensity of the exercise undertaken. Moreover, these hormonal fluctuations appear independent of pituitary control, suggesting a direct relationship between exercise intensity and the observed hormonal responses [22].
This highlights the intricate interplay between physical activity and the endocrine system, with implications for understanding the regulatory mechanisms governing hormonal dynamics during exercise. Consistently, a randomized control trial conducted on 94 postmenopausal women reported that a 12-week exercise program proved to be effective in enhancing estradiol levels among postmenopausal women with osteoporosis. Notably, the efficacy of anaerobic exercise surpassed that of aerobic exercises, demonstrating a more pronounced impact on estradiol levels and lean mass [15].
In contrast, another randomized clinical trial was conducted over a 16-week period, wherein participants engaged in 150 minutes per week of moderate aerobic exercise, revealed no statistically significant alterations in sex hormone levels among young women, including estrogens [23].
Another randomized clinical trial identified a discernible linear dose–response relationship for aerobic exercise, specifically in reducing follicular phase estrogen area under the curve (AUC) among young women. Noteworthy is the absence of any discernible changes in luteal phase estrogen or progesterone levels, indicating a targeted impact of aerobic exercise on the hormonal milieu, specifically during the follicular phase [24].
The third objective explored in this study was to examine the correlation between estrogen levels and HbA1c. The analysis revealed a negative correlation between these two variables, suggesting an inverse relationship. This finding contributes valuable insights into the potential interplay between estrogen levels and HbA1c, adding a nuanced dimension to our understanding of their association. Insulin resistance and estrogen deficiency represent concurrent disorders characterized by a mutually influential relationship [25].
The presence of insulin resistance, coupled with compensatory hyperinsulinemia, triggers an elevation in androgen synthesis at the expense of diminished estrogen production [26]. Likewise, a significant decrease in serum estrogen levels increases the occurrence of insulin-resistant conditions in both males and females [27].
Premenopausal women in good health experience the protective advantages of estrogen, safeguarding them against metabolic and hormonal disruptions. Nevertheless, a subtle decrease in their circulating estrogen levels, coupled with insulin resistance, can heighten the vulnerability to cancers. This risk is particularly notable in organs with a high demand for estrogen, such as the breast, endometrium, and ovary [25, 28].
Our findings were that estrogen (E2) demonstrated a positive correlation with HbA1c in non-diabetic men, while in women, sex hormone-binding globulin exhibited an inverse correlation with HbA1c [29].
The results of this study emphasize the gender-specific connections between hormonal factors and levels of HbA1c, thus enhancing our comprehension of the complex relationship between sex hormones and metabolic parameters. Consistently, a negative correlation between estrogen levels and HbA1c levels was observed among pre-and postmenopausal females, irrespective of DM status. Additionally, diabetic females exhibited a lower mean estrogen level compared to non-diabetic females [30].
Limitations
We encountered certain limitations in our study, notably the adoption of a single-arm design due to challenges in recruiting control participants and convincing them to undergo exercise interventions and blood testing. Another constraint was the use of a relatively small sample size. However, it is essential to note that despite this limitation, the sample size was sufficient to yield the statistical power necessary for effectively addressing the research question.
Conclusion
In conclusion, our study demonstrates that engaging in moderate aerobic exercise effectively enhances glycemic control and reduces insulin resistance, as evidenced by a decrease in daily insulin dose and increasing estrogen levels among young female diabetic patients. These findings underscore the potential benefits of incorporating moderate aerobic exercise into the management strategies for diabetes in this specific demographic.
References
Paschou SA, Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. On type 1 diabetes mellitus pathogenesis. Endocrine connections. 2018;7(1):R38-R46.
Stankov K, Benc D, Draskovic D. Genetic and epigenetic factors in etiology of diabetes mellitus type 1. Pediatrics. 2013;132(6):1112-22.
Patterson CC, Karuranga S, Salpea P, Saeedi P, Dahlquist G, Soltesz G, Ogle GD. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes research and clinical practice. 2019;157:107842. https://doi.org/10.1016/j.diabres.2019.107842.
Kaul K, Apostolopoulou M, Roden M. Insulin resistance in type 1 diabetes mellitus. Metabolism. 2015;64(12):1629-39.
Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine reviews. 2013;34(3):309-38.
Redondo MJ, Libman I, Maahs DM, Lyons SK, Saraco M, Reusch J, Rodriguez H, DiMeglio LA. The evolution of hemoglobin A1c targets for youth with type 1 diabetes: rationale and supporting evidence. Diabetes Care. 2021;44(2):301-12.
Fowler MJ. Microvascular and macrovascular complications of diabetes. Clinical diabetes. 2008;26(2):77-82.
Freeborn D, Dyches T, Roper SO, Mandleco B. Identifying challenges of living with type 1 diabetes: child and youth perspectives. Journal of clinical nursing. 2013;22(13-14):1890-8.
Bernardini AL, Vanelli M, Chiari G, Iovane B, Gelmetti C, Vitale R, Errico MK. Adherence to physical activity in young people with type 1 diabetes. Acta Biomed. 2004;75(3):153-7.
Hall KE, McDonald MW, Grisé KN, Campos OA, Noble EG, Melling CJ. The role of resistance and aerobic exercise training on insulin sensitivity measures in STZ-induced Type 1 diabetic rodents. Metabolism. 2013;62(10):1485-94.
Ramalho AC, de Lourdes Lima M, Nunes F, Cambuí Z, Barbosa C, Andrade A, Viana A, Martins M, Abrantes V, Aragão C. The effect of resistance versus aerobic training on metabolic control in patients with type-1 diabetes mellitus. Diabetes research and clinical practice. 2006;72(3):271-6.
Nojima H, Watanabe H, Yamane K, Kitahara Y, Sekikawa K, Yamamoto H, Yokoyama A, Inamizu T, Asahara T, Kohno N. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2008;57(2):170-6.
Yaribeygi H, Atkin SL, Simental‐Mendía LE, Sahebkar A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. Journal of cellular physiology. 2019;234(8):12385-92.
Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, Chrousos GP, Sidossis LS. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54(11):1472-9.
Razzak ZA, Khan AA, Farooqui SI. Effect of aerobic and anaerobic exercise on estrogen level, fat mass, and muscle mass among postmenopausal osteoporotic females. International journal of health sciences. 2019;13(4):10.
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes care. 2010;33(12):e147-e67.
Kyral AM, Shipherd AM, Hearon CM. The effect of moderate intensity aerobic exercise on affect and exercise intention in active and inactive college students. International journal of exercise science. 2019;12(5):1070.
Tonoli C, Heyman E, Roelands B, Buyse L, Cheung SS, Berthoin S, Meeusen R. Effects of different types of acute and chronic (training) exercise on glycaemic control in type 1 diabetes mellitus: a meta-analysis. Sports medicine. 2012;42:1059-80.
Yardley JE, Kenny GP, Perkins BA, Riddell MC, Balaa N, Malcolm J, Boulay P, Khandwala F, Sigal RJ. Resistance Versus Aerobic Exercise: Acute effects on glycemia in type 1 diabetes. Diabetes Care. 2013;36(3):537-42. https://doi.org/10.2337/dc12-0963.
Gao S, Tang J, Yi G, Li Z, Chen Z, Yu L, Zheng F, Hu Y, Tang Z. The therapeutic effects of mild to moderate intensity aerobic exercise on glycemic control in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Diabetes Therapy. 2021;12:2767-81.
Wong CH, Chiang YC, Wai JPM, Lo FS, Yeh CH, Chung SC, Chang CW. Effects of a home‐based aerobic exercise programme in children with type 1 diabetes mellitus. Journal of clinical nursing. 2011;20(5‐6):681-91.
Jurkowski JE, Jones NL, Walker C, Younglai EV, Sutton JR. Ovarian hormonal responses to exercise. Journal of Applied Physiology. 1978;44(1):109-14.
Smith AJ, Phipps WR, Arikawa AY, O'Dougherty M, Kaufman B, Thomas W, Schmitz KH, Kurzer MS. Effects of aerobic exercise on premenopausal sex hormone levels: results of the WISER study, a randomized clinical trial in healthy, sedentary, eumenorrheic women. Cancer epidemiology, biomarkers & prevention. 2011;20(6):1098-106.
Schmitz KH, Williams NI, Kontos D, Domchek S, Morales KH, Hwang W-T, Grant LL, DiGiovanni L, Salvatore D, Fenderson D. Dose–response effects of aerobic exercise on estrogen among women at high risk for breast cancer: a randomized controlled trial. Breast cancer research and treatment. 2015;154:309-18.
Suba Z. Interplay between insulin resistance and estrogen deficiency as co-activators in carcinogenesis. Pathology & Oncology Research. 2012;18:123-33.
Jelenik T, Roden M. How estrogens prevent from lipid-induced insulin resistance. Oxford University Press; 2013.
De Paoli M, Zakharia A, Werstuck GH. The role of estrogen in insulin resistance: a review of clinical and preclinical data. The American Journal of Pathology. 2021;191(9):1490-8.
Samavat H, Kurzer MS. Estrogen metabolism and breast cancer. Cancer letters. 2015;356(2):231-43.
Xu Y, Cao W, Shen Y, Tang J, Wang Y, Ma X, Bao Y. The relationship between sex hormones and glycated hemoglobin in a non-diabetic middle-aged and elderly population. BMC Endocrine Disorders. 2022;22(1):1-6.
Yadav P, Seth S, Kiranchugh S, Sehgal P. Estradiol levels and their association with type 2 diabetes in North Indian men and women. Int J Gen Med Pharm. 2016;5:7-12.