Research Article - (2023) Volume 18, Issue 6
Combined Lowintensity Laser Therapy And Hyperbaric Oxygen Therapy On Healing Of Chronic Diabetic Foot Ulcers A Controlle
Heba A. Bahey El-Deen1, Amir N Wadee2, Zeezy S. Eraky3*, Haitham M. Elmasry4, Mohamed Salah El- Sayed5 and Siham M Fahmy6*Correspondence: Zeezy S. Eraky, Lecturer of Physical Therapy, Department of Physical Therapy for Internal Medicine and Elderly, Faculty of Physical Therapy, Modern University for Tec, Egypt, Email:
2Professor of Basic Sciences, Faculty of Physical Therapy, Cairo University and Professor of Basic Sciences, Faculty of Physical Therapy, October 6 Uni, Egypt
3Lecturer of Physical Therapy, Department of Physical Therapy for Internal Medicine and Elderly, Faculty of Physical Therapy, Modern University for Tec, Egypt
4Lecturer of Physical Therapy, Department of Basic Science, Faculty of Physical Therapy, Modern University for Technology and Information, Egypt
5Department of Physical Therapy for Pediatrics, Faculty of Physical Therapy, Horus University, New Damietta, Egypt
6Associate Professor of Physical Therapy at Egyptian Chinese University and Military Medical Academy, Consultant Acupuncturist at Military Hospitals, Egypt
Received: 15-Dec-2023 Published: 20-Dec-2023
Abstract
Purpose: This investigation was conducted to evaluate the effectiveness of low-level laser therapy (LLLT) photobio modulation (PBM) and hyperbaric oxygen therapy (HBOT) on healing of prolonged diabetic foot ulcers.
Patients and Methods: One hundred patients with chronic diabetic foot ulcers (DFU); their ages ranged from 40-65 years. The patients were assigned randomly into four groups. Control group received conventional wound care only, LLLT group received GaAlAs diode laser, its power output was1440 mW with following wave lengths: 5 x 850 nm 200 mW, 12 x 670 nm 10mW, 8 x 880 nm 25 mW, 8 x 950nm 15mW and energy density (flounce) was adjusted for 4 J/cm2 with pulse frequency of 10 KHZ, each session lasted 8 minutes for 3 times per week day after day. HBOT group received 100%pure oxygen under 2.5 ATA delivered for 60 minutes per session for 30 sessions with 5 sessions per week for6 successive weeks. Combined group received combination of both LLLT and HBOT. All groups received standard wound care in addition to their program. Measurements for ulcer surface area (USA) by transparent method and ulcer volume (UV) by volumetric method were performed before starting the study, in the second, fourth- and sixth-weeks post treatment.
Results: MANOVA test revealed that there was statistically significant reduction in USA and UV in the LLLT, HBOT and Combined groups (P-value= 001 and 0.0001 in all groups respectively). Regarding USA, there were
insignificant differences among groups in the second, fourth, and sixth weeks (P-value= 0.01 for all measurements). Regarding ulcer volume, there were substantial variations post- 2, 4, and 6-weeks tests (P-value= 0.01, 0.0001, and 0.0001 respectively). Multiple comparison in-between groups showed insignificant differences in-between the experimental groups.
Conclusion: The combination therapy between LLLT does not accelerate the healing rate in chronic DFU more than and LLLT or HBOT alone.
Keywords
low-level laser therapy, Photo bio modulation, Hyperbaric oxygen therapy, Diabetic chronic ulcers.
Introduction
Chronic diabetic foot ulcer (DFU) is a significant issue in the world of health care. Its complications could result in extended hospital stays and amputation. Within the medical community, managing it continues to be a substantial financial and medical challenge [1-6]. The regular stages of healing will be hampered due to the hypoxic nature and subsequent wound infection of DFU, which will finally leave a persistent ulcer [7].
For more efficient wound healing, a number of strategies have been developed, the most crucial of which are glycemic management, wound debridement, antibiotics, regular dressings, and pressure relieving techniques. Physical therapies such low-level laser therapy, hyperbaric oxygen therapy, therapeutic ultrasound, and electromagnetic therapies have been developed as adjuvant treatments for wound healing[13–16].Low-level laser treatment (LLLT) has been noted to promote faster tissue repair, reduced inflammation, regulation of inflammatory mediators, and neovascularization in the healing of wounds [17–19].
It has been demonstrated that oxygen is necessary for the normal wound healing process. This is due to the fact that activities necessary for wound healing, such as fibroblast imitation, collagen deposition, angiogenesis, infection resistance, and intracellular leukocyte bacterial killing, are all oxygensensitive. A systemic treatment approach known as hyperbaric oxygen therapy (HBOT) involves the patient breathing only 100% oxygen for a predetermined amount of time in a pressurized chamber. HBOT has been shown to enhance tissue oxygenation, which aids in the healing of DFUs[20–26].The synergistic effect of LLLT and HBOT may be more successful than each of them separately, according to our study's hypothesis.
Methods and Materials
Design of the study
From May to September 2021, a randomized controlled trial was conducted in the diabetic foot care clinic at Egypt's Kobri El Koba Military Hospital. Human research was conducted in accordance with the Declaration of Helsinki Guidelines for the Conduct of Human Research. After being told about the trial's nature and goal, all patients signed a consent form before data collection began. The CONSORT statement was used to report this study (attach Appendix I: the checklist of CONSORT). Every participant had the option to refuse or withdraw at any time. Through the coding of all data, the confidentiality and anonymity of any obtained information were ensured.
Subjects, Equipment and Procedures
Subjects
All patients were randomly assigned into four groups (control, LLLT, HBOT and combined, each group n=25 patients) using closed envelope method, as shown in Figure 1. Measurement of both ulcer surface area (primary outcome) and ulcer volume (secondary outcome) were performed in the baseline and consequently in the 2nd, 4th, and 6thweektimepoints. Based on non-probability convenience consecutive sampling, one hundred diabetic patients (type 2 diabetes for at least ten years) of both genders (57 males and 43 females) were selected, ranging in age from 40 to 65 years. They were enrolled and their eligibility to participate in the study was determined. All patients were given a thorough examination to confirm that they met the inclusion and exclusion criteria. All patients had a non-infected grade II chronic DFU (duration of ulcer 6 weeks) with good vascularity, as determined by a vascular or general surgeon. All of the patients' blood glucose levels were under control (HbA1C 48 mmol/ mol). All of the patients had a cardiac ejection fraction (CEF) of less than 50% and a blood pressure (BP) of less than 150/90 mmHg. The X-ray of the chest was found to be normal. All patients were clinically assessed by a specialized ENT physician to confirm that they were suitable for the hyperbaric chamber. Patients with chronic obstructive lung disease, patients with any ailment that leads to ulcer other than diabetes, such as chronic venous insufficiency, pregnant and malignant patients undergoing radiotherapy, and patients with cardiac pacemakers were also excluded from the study. The Faculty of Physical Therapy's research Ethical Committee at Cairo University approved the study (redacted) and it was registered on PACTR (redacted) (Figure 1).
Figure 1. Flow chart for the dropout and allocation.
Equipment’s
Laser Cluster Diode: (GaAlAs diode system) Vectra Genisys laser n.27808, Chattanooga group of encores medical, Texas, USA was applied in contact to the wound using cling film as an isolator between the device head and the ulcer site.
Hyperbaric Oxygen Therapy: HAUX-STRACOM 2000/5,5. Double-lock, divided in main chamber and ante chamber / Ω-shape with CE-Certificate, Germany.
Procedures
Testing procedure
Measurement of USA: the patient lied in a comfortable position exposing the affected foot. Two plastic transparent layers were applied on the ulcer to bed demarcated using a fine-tipped permanent marker where one of them is divided into a metric grid of 16x16 squares. The area of each square measures 1 cm2. The transparent sheet that was applied directly to the ulcer was afterwards disinfected to avoid any contamination then was discarded. The transparent sheet was scanned by an HP laser Jet Printer (M1005 MFP). The scanned ulcer was saved as an image using Adobe Photoshop CS6 program to calculate its surface area[27].
Measurement of UV: Saline solution was used to measure the UV, by using 20cm³ syringe filled with saline to fill the cavity of the ulcer. Each patient was positioned to allow complete filling of the ulcer against gravity. An adhesive transparent elastic layer of plastic material was applied tightly over the cleaned ulcer. The saline amount needed to fill the ulcer cavity is a direct measure of ulcer volume[28].
Intervention procedure
The control group received standard wound care, which included wound washing to keep the ulcer surface moist. Place a new bandage on the region after washing it twice a day with saline. There's no need to use hydrogen peroxide or alcohol to clean [29].
LLLT Group: Using a contact technique, 33 diode cluster applicators were employed to deliver LLLT, providing a total power output of 1440 mW with the following wave lengths: 5 x 850nm 200mW lasers, 12 x 670nm 10mW lasers, 8 x 880nm 25 mW lasers, and 8 x 950nm 15mW lasers were used, with an energy density (flounce) of 4 J/cm2 and a pulse frequency of 10 KHZ. Both the therapist and the patient wear sunglasses to protect their eyes. Each session lasted 8 minutes and was held three times each week for a total of six weeks.
HBOT Group: 25 patients got HBO therapy, which began with a gradual increase in oxygen pressure to roughly 2.5 ATA over 15 minutes, followed by 60 minutes of 100% oxygen delivery.
Then, for 30 sessions, gradually decompress for 15 minutes at a time for a total of 90 minutes per session (5 sessions per week for 6 weeks).
Combined Group: HBOT was administered first, followed by LLLT in the same session, with the same parameters as before.
Statistical Analysis
The IBM SPSS version 22 computer application was used to do all statistical calculations (IBM Corporation, USA). The G*Power programme was used to do the sample size calculations (version 3.0.10). The primary outcome measure was the ulcer surface area. The impact of the United States was judged to be moderate (0.25). It would be necessary to generate a sample size of at least 20 patients each group. In order to account for a 20% dropout rate, a total sample size of at least 100 patients was required. The homogeneity test (Leven's test) revealed that all of the data are homogeneous. A normality test (Shapiro-Wilk Test) was done prior to statistical analysis, and the results revealed that the data were normally distributed. To compare data inside and between groups, a parametric test was performed (MANOVA). Post-Hoc was used to discover the least significant difference in repeated comparisons (LSD). A P-value of less than 0.05 was considered statistically significant.
Results
General demographic data
There were statistically insignificant differences between the groups regarding the duration of diabetes, duration of ulcer, initial ulcer surface area, and initial ulcer depth, as illustrated in (Table 1).
Variables |
ControlGroup |
LLLT group |
HBOT group |
Combined group |
P-value |
|---|---|---|---|---|---|
Age(year) |
60.1±9.1 |
59.1±7.68 |
60.9±8.3 |
59.5±8.7 |
0.52 |
DDM(year) |
15.9±3.2 |
16±4.4 |
16.1±3.4 |
16.05±3.8 |
0.47 |
DU(month) |
3.2±1.5 |
3.5±1.7 |
3.3±1.3 |
3.4±1.6 |
0.11 |
IUSA(cm2) |
5.61±0.70 |
5.57±0.70 |
5.48±0.26 |
5.67+0.26 |
0.98 |
IUD (cm3) |
6.10±1.05 |
5.30±0.77 |
7.84±0.75 |
6.60±0.88 |
0.75 |
X± SD: mean± standard deviation. DDM: Duration of Diabetes mellitus.
IUD: Initial Ulcer Duration.IUSA: Initial Ulcer Surface Area.
IUD: Initial Ulcer Depth. Significant at P-value <0.05.
Ulcer surface area (USA)
The within group comparison revealed an insignificant difference in the control group (P-value= 1) while significant differences in LLLT, HBOT, and combination groups (P-value= 0.001 for all groups). The percentage of improvement is higher in the HBOT than in the LTLT and combined groups (89.76%, 85.64%, and 89.42 respectively). The in-between comparison revealed an insignificant difference in pre-test (P-value= 0.25) while significant differences between groups in the second, fourth, and sixth weeks (P-value= 0.01 for all measurements), as clarified in (Table 2 and Figure 2).
|
|
USA |
|||||
|---|---|---|---|---|---|---|
Pre-test |
Post 2 |
Post 4 |
Post 6 |
P-value (Within-Group Comparison) |
Percentage of Improvement |
|
Control |
5.61 + 0.70 |
5.51 + 0.68 |
5.54 + 0.70 |
5.51 + 0.70 |
1 |
2% |
LLLT |
5.57 + 0.70 |
2.98 + 0.35 |
1.42 + 0.28 |
0.8 + 0.30 |
0.001* |
85.64% |
HBOT |
5.86 + 0.26 |
1.86 + 0.24 |
1 + 0.16 |
0.60 + 0.22 |
0.001* |
89.76% |
Combined |
5.67 + 0.26 |
1.66 + 0.23 |
0.92 + 0.18 |
0.60 + 0.23 |
0.001* |
89.42% |
P-value (In-Between Group Comparison) |
0.25 |
0.001* |
0.001* |
0.001* |
|
|
*significant at p-value <0.05
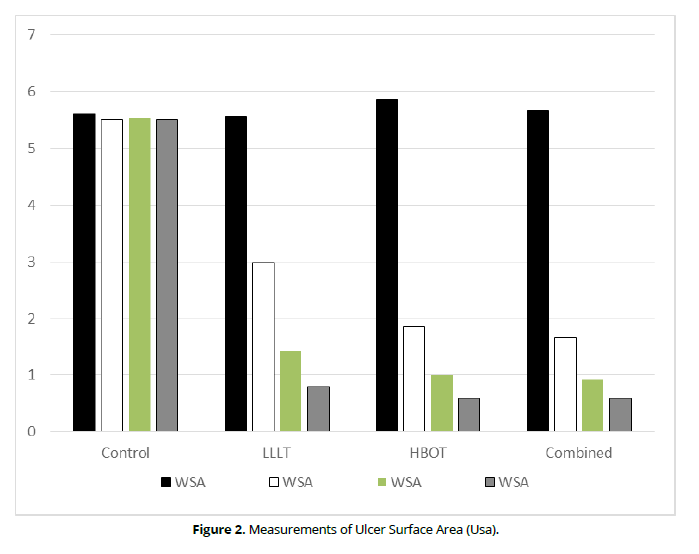
Figure 2. Measurements of Ulcer Surface Area (Usa).
Ulcer volume
The within group comparison revealed an insignificant difference in the control group (P-value= 0.98) significant differences in the LLLT, HBOT and the combined groups (P-value= 0.0001 in all groups). The percentage of improvement is higher in the LILT than the HBOT and Combined groups (89%, 81.12%, and 80.91% respectively). The in-between group comparison showed an insignificant difference in the pre-test (P-value= 0.22) while significant differences post- 2-, 4-, and 6-weeks tests (P-value= 0.01, 0.0001, and 0.0001, respectively, as illustrated in (Table 3 and Figure 3).
|
|
Ulcer Volume |
|||||
|---|---|---|---|---|---|---|
Pre-test |
Post 2 |
Post 4 |
Post 6 |
P-value (Within-Group Comparison) |
Percentage of Improvement |
|
Control |
6.10 +1.05 |
6 + 1.04 |
6.45 + 1.28 |
6.67 + 1.37 |
0.98 |
9.3% |
LILT |
5.30 + 0.77 |
2.85 + 0.46 |
0.81 + 0.23 |
0.56 + 0.29 |
0.0001* |
89.4% |
HBOT |
7.84 + 0.75 |
4.66 + 0.52 |
2.85 + 0.51 |
1.48 + 0.54 |
0.0001* |
81.12% |
Combined |
6.60 + 0.88 |
3.63 + 0.57 |
2.03 + 0.58 |
1.26 + 0.57 |
0.0001* |
80.91% |
P-value (In-Between Group Comparison) |
0.22 |
0.01 |
0.0001 |
0.0001 |
|
|
*significant at p-value <0.05.
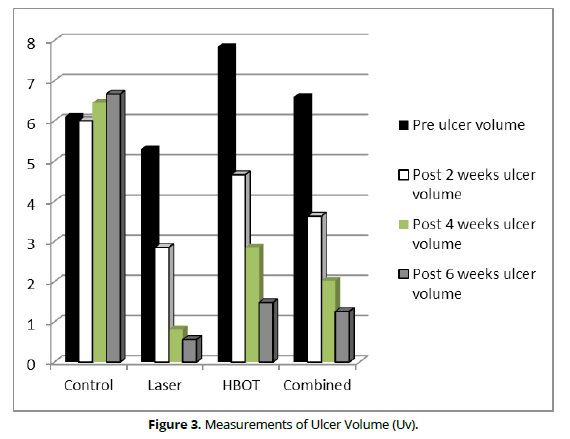
Figure 3. Measurements of Ulcer Surface Area (Usa).
Multiple Comparison within the experimental Groups
There were significant differences between each consequence measurements except between the 4th and 6th week tests, as shown in (Table 4).
WSA |
(I) Groups |
(J) Groups |
Mean Difference (I-J) |
P-value |
Ulcer Volume |
(I) Groups |
(J) Groups |
Mean Difference (I-J) |
P-value |
|---|---|---|---|---|---|---|---|---|---|
LLLT |
Pre-test |
Post 2-weeks |
2.59* |
0.0001 |
LLLT |
Pre-test |
Post 2-weeks |
2.44* |
0.001 |
Post 4-weeks |
4.15* |
0.0001 |
Post 4-weeks |
4.48* |
0.0001 |
||||
Post 6-weeks |
4.71* |
0.0001 |
Post 6-weeks |
4.74* |
0.0001 |
||||
Post 2-weeks |
Post 4-weeks |
1.56* |
0.013 |
Post 2-weeks |
Post 4-weeks |
2.04* |
0.004 |
||
Post 6-weeks |
2.12* |
0.001 |
Post 6-weeks |
2.30* |
0.001 |
||||
Post 4-weeks |
Post 6-weeks |
0.56 |
0.364 |
Post 4-weeks |
Post 6-weeks |
0.26 |
0.706 |
||
HBOT |
Pre-test |
Post 2-weeks |
1* |
0.002 |
HBOT |
Pre-test |
Post 2-weeks |
3.18* |
0.0001 |
Post 4-weeks |
1.86* |
0.0001 |
Post 4-weeks |
4.99* |
0.0001 |
||||
Post 6-weeks |
2.27* |
0.0001 |
Post 6-weeks |
6.36* |
0.0001 |
||||
Post 2-weeks |
Post 4-weeks |
0.85* |
0.009 |
Post 2-weeks |
Post 4-weeks |
1.81* |
0.032 |
||
Post 6-weeks |
1.26* |
0.0001 |
Post 6-weeks |
3.8* |
0.0001 |
||||
Post 4-weeks |
Post 6-weeks |
0.41 |
0.205 |
Post 4-weeks |
Post 6-weeks |
1.37 |
0.103 |
||
Combined |
Pre-test |
Post 2-weeks |
1* |
0.002 |
Combined |
Pre-test |
Post 2-weeks |
2.96* |
0.002 |
Post 4-weeks |
1.75* |
0.0001 |
Post 4-weeks |
4.56* |
0.0001 |
||||
Post 6-weeks |
2.07* |
0.0001 |
Post 6-weeks |
5.33* |
0.0001 |
||||
Post 2-weeks |
Post 4-weeks |
0.75* |
0.023 |
Post 2-weeks |
Post 4-weeks |
1.60 |
.090 |
||
Post 6-weeks |
1.06* |
0.001 |
Post 6-weeks |
2.38* |
0.013 |
||||
Post 4-weeks |
Post 6-weeks |
0.31 |
0.332 |
Post 4-weeks |
Post 6-weeks |
0.77 |
0.412 |
*significant at p-value <0.05; A: Surface area; V: Ulcer volume.
Multiple Comparison In-Between Groups
There were significant differences between the control group and each of the three experimental groups while insignificant differences in-between the experimental groups, as shown in (Table 5).
WSA |
(I) Groups |
(J) Groups |
Mean Difference (I-J) |
P-value |
Ulcer Volume |
(I) Groups |
(J) Groups |
Mean Difference (I-J) |
P-value |
Post 2-weeks |
Control |
LLLT |
2.54* |
0.0001 |
Post 2-weeks |
Control |
LLLT |
3.15* |
0.002 |
HBOT |
3.66* |
0.0001 |
HBOT |
2.35* |
0.016 |
||||
Combined |
3.85* |
0.0001 |
Combined |
2.36* |
0.017 |
||||
LLLT |
HBOT |
1.12 |
0.059 |
LILI |
HBOT |
2.81** |
0.037 |
||
Combined |
1.31* |
0.028 |
Combined |
0.79 |
0.421 |
||||
HBOT |
Combined |
0.19 |
0.745 |
HBOT |
Combined |
2.02* |
0.0418 |
||
Post 4-weeks |
Control |
LLLT |
4.13* |
0.0001 |
Post 4-weeks |
Control |
LLLT |
5.64* |
0.0001 |
HBOT |
4.54* |
0.0001 |
HBOT |
3.61* |
0.001 |
||||
HBOT |
4.63* |
0.0001 |
Combined |
4.42* |
0.0001 |
||||
LLLT |
HBOT |
0.42 |
0.455 |
LLLT |
HBOT |
2.03* |
0.048 |
||
Combined |
0.50 |
0.369 |
Combined |
1.22 |
0.256 |
||||
HBOT |
Combined |
0.08 |
0.879 |
HBOT |
Combined |
3.81* |
0.02 |
||
Post 6-weeks |
Control |
LLLT |
4.65* |
0.0001 |
Post 6-weeks |
Control |
LLLT |
6.01* |
0.0001 |
HBOT |
4.91* |
0.0001 |
HBOT |
5.09* |
0.0001 |
||||
Combined |
4.90* |
0.0001 |
Combined |
5.30* |
0.0001 |
||||
LLLT |
HBOT |
0.26 |
0.657 |
LLLT |
HBOT |
0.92 |
0.418 |
||
Combined |
0.25 |
0.668 |
Combined |
0.71 |
0.534 |
||||
HBOT |
Combined |
0.01 |
0.988 |
HBOT |
Combined |
0.21 |
0.851 |
||
*significant at p-value <0.05.
Discussion
When the four groups were compared in terms of wound surface area and ulcer volume measures, there were no significant differences in pre-treatment values (P= 0.25, 0.22), demonstrating that the four groups were properly matched. The results of the concurrent study showed significant improvements in USA and UV in each of the three intervention groups (P-values of 001 and 0.0001 in each group). In the second, fourth, and sixth weeks of measurements, there were statistically significant differences between the mean values of the examined parameters in the LLLT, HBOT, and combination groups compared to the control group (P-value= 0.001 for all measurements in the USA and 0.01, 0.0001, and 0.0001 for the three UV measurements).
Its photo-biomodulation (PBM), which causes both photo physical and photochemical changes within cells without causing heat damage, may be the cause of the considerable impacts of LLLT on USA and UV. Through the interaction of photons with one or more cellular chromospheres, this PBM induces cellular activity. Cytochrome c oxidase (CCO), the final enzyme in the mitochondrial respiratory chain, is where photons are absorbed as a result of LLLT. Increased ATP synthesis, cellular metabolic activity, and cell proliferation are only a few of the downstream effects of CCO activation [29].
The fact that HBOT significantly increases wound tissue partial pressure of oxygen (PO2) with a mean value of 1.884 in comparison to LLLT with a mean value of 0.265 may account for its effects on USA and UV [30,31].
Following HBOT, vascular endothelial development factor (VEGF), a signalling molecule that encourages the growth of new blood vessels, was found to be present at higher levels, according to histological analysis. When cells and tissues lack oxygenated blood due to decreased blood circulation, signs of enhanced angiogenesis, and more advanced remodelling with higher-quality collagen, VEGF is a component of the system that restores blood flow to them [32, 33]. The HBOT group experienced a greater percentage increase in surface area when compared to the LILT and combination groups (89.76%, 85.64%, and 89.42%, respectively). This was related to HBOT's capacity to reverse local tissue hypoxia, which, by momentarily raising oxygen levels within wounded tissue, induces vasculogenesis and enhances local blood flow. reducing inflammation, preventing the growth of anaerobic bacteria, and facilitating healing [34, 35]
Regarding ulcer volume, LILT group improvement was greater than HBOT and combined group improvement (89.4%, 81.12%, and 80.91%, respectively). There is a lack of recognition and comprehension of the specific mechanisms behind PBM and LILT. However, a wide variety of impacts have been seen at the molecular, cellular, and tissue levels. The presence of cellular mitosis, keratinocyte migration and proliferation, an increase in adenosine triphosphate (ATP), vasodilation, protein creation, a decrease in prostaglandin levels, and neoangiogenesis are all outcomes of its ability to conduct developing mitochondrial activity [36,37].
On the other hand, LLLT parameters like wavelengths (longer wavelengths penetrate tissue much deeper and are used to treat deeper-seated tissues; lower wavelengths treat superficial tissue), power, and dose are crucial, and PBM varies depending on their values [38]. As a result, it may be advised to first use LLLT for a period of time to achieve the best ulcer volume improvement before moving to HBOT for an additional duration of time to achieve the best wound surface area results. The accuracy of this procedure depends on there being no lingering effects from LLLT during the HBOT administration period.
Multiple comparisons within group revealed significant improvement in both ulcer surface area and ulcer volume between each pair of subsequent tests for the LLLT and HBOT groups, with the exception of the period between 4- and 6-weeks. This might be explained by the fact that healing quickened in the first four weeks but slowed down later on. Short-term and long-term PBM effects of LLLT can be traditionally separated. The results, or responses, are what might be seen shortly or immediately after irradiation. Long-term effects are those that appear hours or days after the radiation has stopped and frequently include new cell biosynthesis, especially during the proliferative stage of inflammatory healing [39]. Additionally, this could be explained by the fact that the transcutaneous oxygen tension rises steadily until it reaches aenhanced angiogenesis, and more advanced remodelling with higher-quality collagen, VEGF is a component of the system that restores blood flow to them [32, 33]. The HBOT group experienced a greater percentage increase in surface area when compared to the LILT and combination groups (89.76%, 85.64%, and 89.42%, respectively). This was related to HBOT's capacity to reverse local tissue hypoxia, which, by momentarily raising oxygen levels within wounded tissue, induces vasculogenesis and enhances local blood flow. reducing inflammation, preventing the growth of anaerobic bacteria, and facilitating healing [34, 35]
Regarding ulcer volume, LILT group improvement was greater than HBOT and combined group improvement (89.4%, 81.12%, and 80.91%, respectively). There is a lack of recognition and comprehension of the specific mechanisms behind PBM and LILT. However, a wide variety of impacts have been seen at the molecular, cellular, and tissue levels. The presence of cellular mitosis, keratinocyte migration and proliferation, an increase in adenosine triphosphate (ATP), vasodilation, protein creation, a decrease in prostaglandin levels, and neoangiogenesis are all outcomes of its ability to conduct developing mitochondrial activity [36,37].
On the other hand, LLLT parameters like wavelengths (longer wavelengths penetrate tissue much deeper and are used to treat deeper-seated tissues; lower wavelengths treat superficial tissue), power, and dose are crucial, and PBM varies depending on their values [38]. As a result, it may be advised to first use LLLT for a period of time to achieve the best ulcer volume improvement before moving to HBOT for an additional duration of time to achieve the best wound surface area results. The accuracy of this procedure depends on there being no lingering effects from LLLT during the HBOT administration period. Multiple comparisons within group revealed significant improvement in both ulcer surface area and ulcer volume between each pair of subsequent tests for the LLLT and HBOT groups, with the exception of the period between 4- and 6-weeks. This might be explained by the fact that healing quickened in the first four weeks but slowed down later on. Short-term and long-term PBM effects of LLLT can be traditionally separated. The results, or responses, are what might be seen shortly or immediately after irradiation. Long-term effects are those that appear hours or days after the radiation has stopped and frequently include new cell biosynthesis, especially during the proliferative stage of inflammatory healing [39]. Additionally, this could be explained by the fact that the transcutaneous oxygen tension rises steadily until it reaches a plateau in accordance with the DFU's oxygen capacity, which is determined by the health of the macro vascular and micro vascular systems, which act as barriers to the systemic distribution of oxygen [40,41].
The combined treatment showed effective results in terms of ulcer surface area from 2- to 4-weeks and 2- to 6-weeks. Hyperbaric oxygen therapy, which has been associated with a quicker closure of surface area, may be to blame for this. In contrast, there was no improvement in ulcer volume between 2- and 4-weeks or 4- to 6-weeks, despite their being an improvement between 2- and 6-weeks. This might be caused by how LLLT affects ulcer volume healing by enhancing collagen production, which happens via PBM pathways on which specific frequencies or doses may act, moderating cellular proliferation, and boosting the levels of fibroblast growth factors. According to the authors mentioned above, another possibility is that the mitochondria take in more energy, leading to increased nucleic acid production, which enhances collagen production, hastens epithelial repair, and promotes the creation of granulation tissue [42].
It's possible that the lack of improvement between weeks 2 and 4 and 4 and 6 is related to the time needed for the chemical reactions brought on by LLLT, which promotes cellular metabolism and increases generation of adenosine triphosphate (ATP), to become established. It works by lowering edoema and oxidative stress [43,44].Therefore, we speculate that LLLT may have an aftereffect in which the healing process persisted even after the end of sessions. Therefore, additional research may be required to demonstrate the real follow-up period.
Insignificant differences were found when comparing the three intervention groups, but significant differences were found when comparing the control group and each of the three intervention groups regarding both USA and ulcer volume at the second, fourth, and sixth weeks. According to these findings, any one of the three intervention methods can be utilized in place of the others, taking into account their safety, cost-effectiveness, and time requirements.
The HBO device has various precautions and contraindications, may require significant space that may not be available in all healthcare institutions, and is therefore unsuitable for all patients. Although potential side effects and consequences must be taken into account, the most frequent HBOT side effect is middle ear barotrauma. Patients with the common cold and upper respiratory tract infections are not candidates for HBOT because the Paranasal sinuses are frequently impacted by barotrauma. Due to the vasoconstrictive effects of oxygen, HBOT also causes an increase in total peripheral resistance, which results in a reduction in heart function [7,33].Additionally, LLLT has the potential to develop into a portable, minimally invasive, simple-to-use, and affordable therapy option for DFU, according to a systematic review [43,44].
Finally, according to the results of this concurrent study, the hypothesis of synergistic effect of LILT and HBOT may be more effective than either of them alone was rejected.
Implications of Physiotherapy Practice
It is concluded that either LLLT or HBOT can be used alone to accelerate healing in chronic DFU. While combined therapy failed to meet the expected results. Further study may be needed to calculate the actual duration where the effect of LLLT lasts.
Funding
• The authors stated and declared that No funders of the study.
Conflicts of interest/Competing interests
• The authors stated and declared that No conflict or competing of interests.
Ethics approval
• The Faculty of Physical Therapy's research Ethical Committee at Cairo university approved the study (No: P.T.RE/003/002020) and it was registered on PACTR (PACTR202008507043910).
Consent to participate
• No experimental investigation was performed on individuals within this research study.
Consent for publication
• All the co-authors are agreed for the research study publication.
Availability of data and material
• The authors stated and declared that all data is exist and available.
Code availability
• The authors stated and declared that all code is exist and available. Permission to reproduce material from other sources
Allowed on request
References
B.A. Lipsky, A.R. Berendt, P.B. Cornia, J.C. Pile, E.J.G. Peters, D.G. Armstrong, H. Gunner Deery, J.M. Embil, W.S. Joseph, A.W. Karchmer, M.S. Pinzur, E. Senneville, 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections, J. Am. Podiatr. Med. Assoc. 103 (2013) 2–7. https://doi.org/10.7547/1030002.
B.A. Lipsky, A.R. Berendt, P.B. Cornia, J.C. Pile, E.J.G. Peters, D.G. Armstrong, H.G. Deery, J.M. Embil, W.S. Joseph, A.W. Karchmer, M.S. Pinzur, E. Senneville, 2012 infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections, Clin. Infect. Dis. 54 (2012) 132–173. https://doi.org/10.1093/cid/cis346.
E.N. Hokkam, Assessment of risk factors in diabetic foot ulceration and their impact on the outcome of the disease, Prim. Care Diabetes. 3 (2009) 219–224. https://doi.org/10.1016/j.pcd.2009.08.009.
B.M. Kajagar, A.S. Godhi, A. Pandit, S. Khatri, Efficacy of Low Level Laser Therapy on Wound Healing in Patients with Chronic Diabetic Foot Ulcers-A Randomised Control Trial, Indian J. Surg. 74 (2012) 359–363. https://doi.org/10.1007/s12262-011-0393-4.
M.K. Yosuf, S.I. Mahadi, S.M. Mahmoud, A.H. Widatalla, M.E. Ahmed, Diabetic neuropathic forefoot and heel ulcers: Management, clinical presentation and outcomes, J. Wound Care. 24 (2015) 420–425. https://doi.org/10.12968/jowc.2015.24.9.420.
O.A. Ogbera, E. Osa, A. Edo, E. Chukwum, Common clinical features of diabetic foot ulcers: Perspectives from a developing nation, Int. J. Low. Extrem. Wounds. 7 (2008) 93–98. https://doi.org/10.1177/1534734608318236.
F. Öztürk, A. Türel Ermertcan, I. İnanır, Hyperbaric oxygen therapy for the management of chronic wounds, Cutan. Ocul. Toxicol. 32 (2013) 72–77.
R. Samaneh, Y. Ali, J. Mostafa, N.A. Mahmud, R. Zohre, Laser therapy for wound healing: A review of current techniques and mechanisms of action, Biosci, Biotech Res Asia. 12 (2015) 217–223.
D.J. Bakker, Hyperbaric oxygen therapy and the diabetic foot, Diabetes. Metab. Res. Rev. 16 (2000) S55–S58.
N. Oliveira, P. Rosa, L. Borges, E. Dias, F. Oliveira, I. Cássio, Treatment of diabetic foot complications with hyperbaric oxygen therapy: a retrospective experience, Foot Ankle Surg. 20 (2014) 140–143.
V.S. Potula, Conventional treatment versus vacuum therapy for diabetic foot ulcers treatment, Int. Surg. J. 5 (2017) 49–53.
S. Van Asten, P. Kim, J. La Fontaine, L.A. Lavery, Diabetic foot ulcers, Wound Care Essentials Pract. Princ. Fourth Ed. 12 (2015) 319–325.
Z. Landau, Topical hyperbaric oxygen and low energy laser for the treatment of diabetic foot ulcers, Arch. Orthop. Trauma Surg. 117 (1998) 156–158. https://doi.org/10.1007/s004020050217.
Z. Landau, A. Sommer, E.B. Miller, Topical hyperbaric oxygen and low-energy laser for the treatment of chronic ulcers, Eur. J. Intern. Med. 17 (2006) 272–275.
C.-J. Wang, R.-W. Wu, Y.-J. Yang, Treatment of diabetic foot ulcers: a comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy, Diabetes Res. Clin. Pract. 92 (2011) 187–193.
F.G. Basso, C.F. Oliveira, C. Kurachi, J. Hebling, C.A. de Souza Costa, Biostimulatory effect of low-level laser therapy on keratinocytes in vitro, Lasers Med. Sci. 28 (2013) 367–374.
F.F. Fathabadie, M. Bayat, A. Amini, M. Bayat, F. Rezaie, Effects of pulsed infra-red low level-laser irradiation on mast cells number and degranulation in open skin wound healing of healthy and streptozotocin-induced diabetic rats, J. Cosmet. Laser Ther. 15 (2013) 294–304.
A.L. Whinfield, I. Aitkenhead, The light revival: does phototherapy promote wound healing? A review, Foot. 19 (2009) 117–124.
M. Pakkiriappan, M. Velayutham, S.M. Karthikeyan, Low level red laser therapy using wavelength 635nm for diabetic foot ulcers: a prospective study, Int. Surg. J. 6 (2019) 527–530.
L. Kessler, P. Bilbault, F. ORTega, C. Grasso, R. Passemard, D. Stephan, M. Pinget, F. Schneider, Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study, Diabetes Care. 26 (2003) 2378–2382.
A. Abidia, G. Laden, G. Kuhan, B.F. Johnson, A.R. Wilkinson, P.M. Renwick, E.A. Masson, P.T. McCollum, The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial, Eur. J. Vasc. Endovasc. Surg. 25 (2003) 513–518.
L. Ma, P. Li, Z. Shi, T. Hou, X. Chen, J. Du, A prospective, randomized, controlled study of hyperbaric oxygen therapy: effects on healing and oxidative stress of ulcer tissue in patients with a diabetic foot ulcer., Ostomy. Wound. Manage. 59 (2013) 18–24.
M. Löndahl, P. Katzman, A. Nilsson, C. Hammarlund, Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes, Diabetes Care. 33 (2010) 998–1003.
R.C. Barnes, Point: hyperbaric oxygen is beneficial for diabetic foot wounds, Clin. Infect. Dis. 43 (2006) 188–192.
E.T. Huang, A clinical practice guideline for the use of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers, (2015).
S.E. Salama, A.E. Eldeeb, A.H. Elbarbary, S.E. Abdelghany, Adjuvant Hyperbaric Oxygen Therapy Enhances Healing of Nonischemic Diabetic Foot Ulcers Compared With Standard Wound Care Alone, Int. J. Low. Extrem. Wounds. 18 (2019) 75–80. https://doi.org/10.1177/1534734619829939.
P.-N. Li, H. Li, M.-L. Wu, S.-Y. Wang, Q.-Y. Kong, Z. Zhang, Y. Sun, J. Liu, D.-C. Lv, A cost-effective transparency-based digital imaging for efficient and accurate wound area measurement, PLoS One. 7 (2012) e38069.
W. Berg, C. Traneroth, A. Gunnarson, C. Lossing, A method for measuring pressure sores, Lancet. 335 (1990) 1445–1446.
H. Chung, T. Dai, S.K. Sharma, Y.-Y. Huang, J.D. Carroll, M.R. Hamblin, The nuts and bolts of low-level laser (light) therapy, Ann. Biomed. Eng. 40 (2012) 516–533.
F. Gottrup, J. Dissemond, C. Baines, R. Frykberg, P.Ø. Jensen, J. Kot, K. Kröger, P. Longobardi, Use of oxygen therapies in wound healing: focus on topical and hyperbaric oxygen treatment, J. Wound Care. 26 (2017) S1–S43.
S.M. Fahmy, M.H. Aref, I.H. Aboughaleb, M. Rabie, R. Abdlaty, Hyperbaric Oxygen Therapy for Healing Diabetic Lower Extremity Ulcers, 2020 12th Int. Conf. Electr. Eng. ICEENG 2020. (2020) 135–139. https://doi.org/10.1109/ICEENG45378.2020.9171697.
E. Huang, M. Heyboer 3rd, D.J. Savaser, Hyperbaric oxygen therapy for the management of chronic wounds: patient selection and perspectives, Chronic Wound Care Manag. Res. 6 (2019) 27–37.
W.J. Ennis, E.T. Huang, H. Gordon, Impact of hyperbaric oxygen on more advanced wagner grades 3 and 4 diabetic foot ulcers: matching therapy to specific wound conditions, Adv. Wound Care. 7 (2018) 397–407.
P.C.L. Silveira, L.A. da Silva, C.A. Pinho, P.S. De Souza, M.M. Ronsani, D. da Luz Scheffer, R.A. Pinho, Effects of low-level laser therapy (GaAs) in an animal model of muscular damage induced by trauma, Lasers Med. Sci. 28 (2013) 431–436.
C.-Y. Chen, R.-W. Wu, M.-C. Hsu, C.-J. Hsieh, M.-C. Chou, Adjunctive hyperbaric oxygen therapy for healing of chronic diabetic foot ulcers, J. Wound, Ostomy Cont. Nurs. 44 (2017) 536–545.
N.N. Houreld, R.T. Masha, H. Abrahamse, Low‐intensity laser irradiation at 660 nm stimulates cytochrome c oxidase in stressed fibroblast cells, Lasers Surg. Med. 44 (2012) 429–434.
R.T. Masha, N.N. Houreld, H. Abrahamse, Low-intensity laser irradiation at 660 nm stimulates transcription of genes involved in the electron transport chain, Photomed. Laser Surg. 31 (2013) 47–53.
H.B. EL-DEEN, S. Fahmy, S.A. Ali, W.M. El-Sayed, Polarized light versus light-emitting diode on healing of chronic diabetic foot ulcer, Rom. J. Biophys. 24 (2014) 1–15.
F. do S. da S.D. Andrade, R.M. de O. Clark, M.L. Ferreira, Effects of low-level laser therapy on wound healing, Rev. Col. Bras. Cir. 41 (2014) 129–133.
S.K. Dogan, S. Ay, D. Evcik, The effectiveness of low laser therapy in sub acromial impingement syndrome: a randomized placebo controlled double-blind prospective study, Clinics. 65 (2010) 1019–1022.
P.D. Hayes, N. Alzuhir, G. Curran, I.M. Loftus, Topical oxygen therapy promotes the healing of chronic diabetic foot ulcers: a pilot study, J. Wound Care. 26 (2017) 652–660.
K.M. AlGhamdi, A. Kumar, N.A. Moussa, Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells, Lasers Med. Sci. 27 (2012) 237–249.
P. Lau, N. Bidin, G. Krishnan, S.M. AnaybBaleg, M.B.M. Sum, H. Bakhtiar, Z. Nassir, A. Hamid, Photobiostimulation effect on diabetic wound at different power density of near infrared laser, J. Photochem. Photobiol. B Biol. 151 (2015) 201–207.
B. Nteleki, N.N. Houreld, The use of phototherapy in the treatment of diabetic ulcers, J. Endocrinol. Metab. Diabetes South Africa. 17 (2012) 128–132.