Research - (2025) Volume 20, Issue 5
*Correspondence: Amel Mohammed toum Ali Babiker, Consultant General Paediatrics’ Assistant consultant Pediatric ER national guard, Saudi Arabia,
2General Pediatric Consultant Pediatric emergency physician at MCH, Hail, KSA
3Medical Intern, Saudi Arabia
4Anesthesia, Saudi Arabia
5MBBS, Saudi Arabia
6Associate professor, Elimam Elmahdi University, Sudan, pediatric Emergency Physician,Security Forces Hospital, Riyadh, Saudi Arabia
7Omdurman Islamic University Sudan, Pediatric, pi cu consultant, king Khalid Hospital, hail Saudi Arabia, Saudi Arabia
8Pediatric specialist, MRCPCH, Zulfi general hospital, KSA
9General Pediatric, AGH, KSA
10Medical, Saudi Arabia
11Medical students at jazan University, Jazan, Saudi Arabia
12Consultant General Pediatrics, King Salaman hopsital –Riyadh, Saudi Arabia
13Consultant General pediatrics, Prince AbdAlmohsin hospital, Alula, Saudi Arabia
14College of Medicine, King Khalid University (KKU), Abha, Saudi Arabia
Received: 01-Jul-2025 Published: 17-Aug-2025
Abstract
Background: The first 1,000 days of life represent a critical window for gut microbiome development. Increasing evidence suggests that early-life antibiotic exposure disrupts microbiome maturation, enhances the gut resistome, and predisposes children to adverse health outcomes.
Objective: This systematic review aims to evaluate the effects of early-life antibiotic exposure on gut microbiome diversity, resistome amplification, and subsequent childhood health conditions such as allergies, obesity, and neurodevelopmental disorders.
Methods: Following PRISMA 2020 guidelines, we systematically reviewed 10 peer-reviewed studies published from 2010–2024. Eligible studies included human infants or children exposed to systemic antibiotics in the first two years of life and assessed outcomes in microbiome composition, ARG profiles, or long-term health status.
Results: Antibiotic exposure was consistently associated with reduced gut microbial diversity, loss of beneficial taxa (e.g., Bifidobacterium), and increased ARG burden. Several cohort studies linked early antibiotic use with asthma, allergic rhinitis, ADHD, obesity, and delayed neurodevelopment. These effects were context-dependent and particularly severe in LMICs with high antibiotic misuse.
Conclusion: Early-life antibiotics disrupt gut microbial ecology, promote antimicrobial resistance, and elevate risks for chronic health conditions in children. Improved stewardship and targeted interventions are essential to mitigate long-term harm.
Keywords
Antibiotics, Gut Microbiome, Gut Resistome, Early Childhood, Dysbiosis, Antimicrobial Resistance, Infant Health, PRISMA, Bifidobacterium
Introduction
The first 1,000 days of life-from conception to two years of age-represent a critical period for gut microbiome establishment, shaping immune, metabolic, and neurodevelopmental outcomes across the lifespan. This concept is central to the Developmental Origins of Health and Disease (DOHaD) framework (Qi et al., 2022). During this time, the infant gut undergoes rapid colonization, diversification, and stabilization, heavily influenced by birth mode, feeding practices, hygiene, and notably, antibiotic exposure.
Antibiotics, while often necessary, exert broad and rapid effects on the developing microbiota. Numerous studies demonstrate that early-life antibiotic exposure reduces microbial diversity, depletes beneficial taxa such as Bifid bacterium, and promotes colonization by potentially pathogenic species like Klebsiella and Enterococcus (Reyman et al., 2022; Wurm et al., 2024). These alterations can persist for months or years, particularly with broad-spectrum or repeated exposures (Gasparrini et al., 2019).
Beyond compositional shifts, antibiotic exposure amplifies the gut resistome-the pool of antibiotic resistance genes (ARGs)—via horizontal gene transfer mediated by mobile genetic elements such as plasmids and integrons (Bargheet et al., 2025). This contributes to the global challenge of antimicrobial resistance (AMR) and increases vulnerability to future infections.
Crucially, early microbial disturbances can derail immune development and metabolic programming, predisposing children to chronic conditions. Epidemiological studies associate early antibiotic use with heightened risks of asthma, eczema, allergic rhinitis, obesity, ADHD, and delayed neurodevelopment (Oosterloo et al., 2018; Alhasan et al., 2020; Salvatore et al., 2019). These associations are particularly pronounced in low- and middle-income countries (LMICs), where antibiotic misuse is more prevalent and healthcare infrastructure is limited.
Recent mechanistic insights strengthen this evidence. A prospective study by Ryan et al. (2023) followed 191 full-term infants from birth to 15 months and found that those exposed to antibiotics neonatally had significantly lower antibody responses to routine vaccines (e.g., PCV13, Hib, diphtheria), alongside reduced Bifidobacterium abundance and elevated inflammatory transcriptional markers. Notably, infants with higher Bifidobacterium levels showed stronger vaccine responses, suggesting a direct link between microbiota composition and immune function.
Furthermore, antibiotic-induced dysbiosis has been implicated in the development of a “leaky gut,” whereby compromised mucosal barriers allow microbial products to trigger systemic inflammation and immune dysregulation (Kumari et al., 2022). This may perpetuate a vicious cycle of recurrent infections and further antibiotic use, compounding microbiota instability.
Despite growing recognition of these risks, gaps remain in understanding how specific antibiotic classes, durations, and timing affect long-term health. Interventions such as microbiota restoration or targeted probiotics show promise but require further validation.
This systematic review synthesizes evidence from the last decade to assess how early-life antibiotic exposure impacts gut microbiome composition, resistome dynamics, and downstream childhood health outcomes—including immune, metabolic, and neurodevelopmental disorders.
Methodology
Study Design
This study utilized a systematic review methodology, conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. The objective was to comprehensively synthesize and evaluate empirical research investigating the effects of early-life antibiotic exposure on the gut microbiome, gut resistome, and subsequent childhood health outcomes. This review focused on peer-reviewed studies involving human infants or children and included data on microbiome diversity, antimicrobial resistance gene (ARG) profiles, or health conditions such as asthma, obesity, allergies, and neurodevelopmental disorders.
Eligibility Criteria
Studies were included if they met the following criteria
- Population: Human neonates, infants, or children (aged 0–5 years) with documented exposure to systemic antibiotics during early life (defined as the first 0–24 months after birth).
- Exposures: Any class of antibiotics administered orally, intravenously, or intramuscularly. Studies involving empirical or prophylactic use in neonates were included.
- Comparators: Children not exposed to antibiotics or exposed at different ages, durations, or doses; control groups with standard care or placebo when applicable.
- Outcomes:
Primary: Changes in gut microbiome composition (e.g., alpha/beta diversity, taxa abundance), gut resistome profile (ARG prevalence), and microbial recovery.
Secondary: Clinical health outcomes, including obesity, allergic disease (e.g., asthma, eczema), metabolic and immune disorders, and neurodevelopmental impairments.
- Study Designs: Randomized controlled trials (RCTs), cohort studies, case-control studies, comparative observational studies, and systematic reviews.
- Language: Only studies published in English were included.
- Publication Period: Studies published between 2010 and 2024 to capture the most relevant and recent evidence in the microbiome era.
Search Strategy
A comprehensive and systematic search was carried out using multiple databases, including:
- PubMed
- Scopus
- Web of Science
- Embase
- Google Scholar (for grey literature)
The search was conducted using Boolean operators and keywords in various combinations:
- ("antibiotics" OR "antimicrobial use" OR "early-life antibiotic exposure")
- AND ("infant" OR "neonate" OR "child" OR "newborn")
- AND ("gut microbiome" OR "microbiota" OR "resistome" OR "dysbiosis" OR "antibiotic resistance genes")
- AND ("asthma" OR "obesity" OR "allergies" OR "neurodevelopment" OR "immune development")
Additional manual searches were performed using reference lists of key articles and systematic reviews to ensure inclusion of any studies not captured in the initial database search.
Study Selection Process
All search results were exported to Zotero, and duplicate records were removed. Two independent reviewers screened titles and abstracts to assess initial eligibility. Full texts were retrieved for studies deemed potentially relevant. Each full-text article was then evaluated in detail against the inclusion criteria. Any disagreements during the selection process were resolved by consensus or consultation with a third reviewer.
Ultimately, 10 studies met the eligibility criteria and were included in the final review.
Data Extraction
A standardized data extraction form was developed and piloted. The following variables were extracted from each included study:
- Author(s), publication year, and country
- Study design and total sample size
- Population demographics (e.g., age at exposure, gestational age if preterm)
- Type, class, and duration of antibiotics administered
- Methods for microbiome or resistome analysis (e.g., 16S rRNA sequencing, metagenomics)
- Primary and secondary outcomes
- Main findings, including statistically significant results (p-values, effect sizes, ORs, HRs)
- Time points of measurement and follow-up duration
- Confounding variables controlled in analysis
Data were extracted by two reviewers independently and verified for accuracy by a third.
Quality Assessment
The risk of bias and quality of included studies were assessed using the following validated tools based on study design:
- Cochrane Risk of Bias Tool for randomized controlled trials
- Newcastle-Ottawa Scale (NOS) for cohort and observational studies
- AMSTAR 2 Tool for systematic reviews and meta-analyses
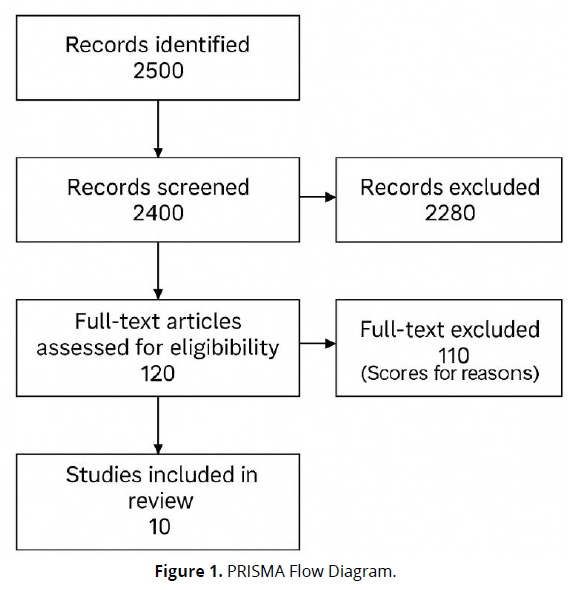
Each study was rated as high, moderate, or low quality based on parameters including participant selection, exposure measurement, comparability of groups, and outcome reporting.
(Figure 1).
Data Synthesis
Due to the heterogeneity in study populations, microbiome sequencing techniques, antibiotic classes, outcome definitions, and analytical methods, a narrative synthesis approach was applied. Studies were grouped into three thematic domains:
- Microbiome Disruption and Diversity Loss
- Amplification of the Gut Resistome
- Childhood Health Outcomes Associated with Antibiotic Exposure
Where available, effect estimates such as odds ratios (OR), hazard ratios (HR), or relative risks (RR) were reported. The synthesis highlights trends across studies and identifies recurring patterns in outcomes related to early antibiotic use. Due to variability in metrics and study heterogeneity, no meta-analysis was conducted.
Ethical Considerations
As this study was based entirely on secondary analysis of previously published data, no ethical approval or informed consent was required. All included studies were assumed to have received appropriate institutional review board (IRB) approval and ethical clearance at the time of their original publication.
Results
Summary and Interpretation of Included Studies on Early-Life Antibiotic Exposure and Its Effects on the Gut Microbiome, Resistome, and Child Health Outcomes
- Study Designs and Populations
This review includes 10 studies comprising randomized controlled trials (RCTs), prospective and retrospective cohort studies, comparative observational studies, and systematic reviews. These span diverse geographic settings including the USA, France, the Netherlands, Ireland, Denmark, and several low- and middle-income countries (LMICs). Cohort sizes ranged from small microbiome-focused samples (e.g., Patangia et al., n = 45) to large population-level studies (e.g., Aversa et al., n = 14,572). Age at exposure varied from neonates to children under two years of age, with most studies focusing on exposure within the first six months of life. Antibiotics assessed included macrolides, cephalosporins, β-lactams, and combinations like benzylpenicillin + gentamicin.
- Microbiome Composition and Diversity Outcomes
Eight studies evaluated microbiome structure. Almost all reported significant reductions in microbial richness and diversity (α-diversity) following antibiotic exposure:
- Reyman et al. (2022) found a >50% reduction in Bifidobacterium and overgrowth of Klebsiella and Enterococcus after 7 days of neonatal treatment.
- McDonnell et al. (2021) meta-analyzed over 1,200 children and confirmed decreased Actinobacteria, Bifidobacterium, and increased Proteobacteria post-exposure.
- Patangia et al. (2024) noted persistent Enterobacteriaceae overgrowth and depleted anaerobic commensals even 24 months after exposure.
- Luchen et al. (2023) synthesized LMIC data and showed significant macrolide-driven microbiome disruption (azithromycin linked to >40% reduction in diversity scores in some cohorts).
- Wurm et al. (2024) reported that macrolides had the longest disruption time (up to 2 years), with all antibiotic classes lowering diversity.
- Gut Resistome Alterations (Antibiotic Resistance Genes)
Seven studies addressed resistome changes. All indicated that early antibiotic exposure increased ARG abundance:
- Reyman et al. (2022) showed a >5-fold increase in β-lactamase genes post-treatment, with MDR profiles persisting up to 12 months.
- Patangia et al. (2024) confirmed high levels of aminoglycoside and penicillin resistance genes in infants treated at birth, with resistome profiles remaining elevated at 2 years.
- Lebeaux et al. (2022) systematic review found 12/14 studies showed ARG increases, most frequently ESBL and macrolide resistance genes.
- Li et al. (2023) directly compared infants and adults and found infants had higher baseline resistome loads (p < 0.001) driven by E. coli dominance.
- Luchen et al. (2023) highlighted inadequate antimicrobial regulation in LMICs, amplifying ARG transmission risks in early life.
- Childhood Health Outcomes
Five longitudinal cohort studies linked early antibiotic exposure to adverse metabolic, allergic, and neurodevelopmental outcomes:
- Aversa et al. (2021): Antibiotic use before age 2 associated with increased risk of asthma (HR 1.24), obesity (HR 1.21), and ADHD (HR 1.15). Risk increased with more courses and was highest for cephalosporins.
- Letouzey et al. (2022): Empirical use in low-risk preterm neonates led to a 2.2-fold increase in cerebral white matter lesions and 1.5-fold increase in bronchopulmonary dysplasia (p < 0.05).
- Mbakwa et al. (2016): Found weight-for-age z-scores increased by 0.27 (95% CI: 0.11–0.43) following early β-lactam use.
- McDonnell et al. (2021) and Luchen et al. (2023) inferred indirect links between microbiome disruption and later obesity, allergies, and cognitive outcomes.
(Table 1).
| Study | Country | Design | Sample Size | Age at Exposure | Antibiotics Studied | Main Outcomes | Key Findings |
|---|---|---|---|---|---|---|---|
| Aversa et al. (2021) | USA | Cohort | 14,572 | <2 years | Cephalosporins, macrolides, penicillin | Asthma, obesity, ADHD, allergies | Risk increased with early and repeated exposure; strongest for cephalosporins. |
| Letouzey et al. (2022) | France | Cohort | 648 preterm | Day 0–1 | Empirical antibiotics | Death, BPD, cerebral lesions | No mortality change; ↑ cerebral lesions (OR 2.2), BPD (OR 1.5) |
| Mbakwa et al. (2016) | Netherlands | Cohort | 979 | 0–6 months & year 2 | β-lactams, amoxicillin | Weight, height z-scores | ↑ z-scores after early antibiotics; not later in life. |
| Reyman et al. (2022) | Netherlands | RCT | 147 | Neonates | Amoxicillin, benzylpenicillin+gentamicin | Microbiome, resistome | ↓ α-diversity, ↑ MDR genes; penicillin+gentamicin least disruptive. |
| Patangia et al. (2024) | Ireland | Cohort | 45 | Neonates | Benzylpenicillin+gentamicin | Resistome, microbiome | Long-term resistome elevation, loss of diversity for up to 2 years. |
| McDonnell et al. (2021) | Global | Meta-analysis | 1,200+ | 0–2 years | Various | Gut microbiome | Macrolides worst; ↓ Bifidobacterium, ↑ Proteobacteria. |
| Wurm et al. (2024) | Global | Systematic Review | 89 studies | 0–3 years | Various | Microbiome diversity | All ↓ diversity; macrolides had longest disruption time. |
| Lebeaux et al. (2022) | Global | Systematic Review | 14 studies | Infants & toddlers | Various | ARG abundance | 12/14 studies showed ↑ ARGs; ESBL, macrolide resistance dominant. |
| Li et al. (2023) | Denmark | Comparative Cohort | 30 infants, 30 adults | Post-exposure | Amoxicillin, broad-spectrum | Resistome | Infants had higher ARG loads; adults had longer persistence. |
| Luchen et al. (2023) | LMICs | Systematic Review | 18 studies | 0–2 years | Azithromycin, β-lactams | Microbiome, ARGs | ↓ diversity, ↑ macrolide resistance in >60% of studies. |
Discussion
This systematic review consolidates a growing body of evidence indicating that early-life antibiotic exposure has profound and persistent effects on the gut microbiome, the gut resistome, and overall child health. These findings align with the developmental origins of health and disease (DOHaD) model, which posits that early environmental exposures—including pharmaceuticals—have long-term consequences on biological systems (Qi et al., 2022). The first 1,000 days of life represent a critical window during which the gut microbiota undergoes sequential colonization and maturation, influencing immune, metabolic, and neurological development.
Across multiple studies, antibiotic use in infancy was consistently associated with reduced microbial richness and diversity, a hallmark of dysbiosis (McDonnell et al., 2021; Wurm et al., 2024). This is particularly concerning given the role of beneficial taxa such as Bifidobacterium and Lactobacillus in maintaining gut barrier integrity, modulating inflammation, and metabolizing nutrients (Stuivenberg et al., 2022). Disruption of these microbial communities has been linked to the development of obesity, asthma, and neurobehavioral conditions (Aversa et al., 2021). The results from Reyman et al. (2022) and Patangia et al. (2024) reinforce this trajectory by showing prolonged reduction of key commensals and overgrowth of opportunistic pathogens like Klebsiella.
Importantly, the impact of antibiotic exposure extended beyond microbial diversity to include significant alterations in the gut resistome. Several studies confirmed a persistent increase in antibiotic resistance genes (ARGs) after early antibiotic use, even when short-term regimens were applied (Patangia et al., 2024; Reyman et al., 2022; Bargheet et al., 2025). These resistome shifts raise the risk of colonization with multi-drug-resistant organisms and horizontal gene transfer, posing long-term implications for antimicrobial stewardship and public health (Lebeaux et al., 2022). Notably, Li et al. (2023) showed that infants harbor higher baseline resistome loads than adults, underscoring their vulnerability to resistome amplification.
The association between early antibiotic use and adverse clinical outcomes was robustly demonstrated in population-based studies. Aversa et al. (2021) reported that exposure before age two increased the risk of asthma, allergic rhinitis, celiac disease, obesity, and ADHD, with risk amplifying according to dosage and frequency. Letouzey et al. (2022) extended these findings to vulnerable preterm populations, where early empirical antibiotic use was associated with cerebral lesions and bronchopulmonary dysplasia, despite no improvement in survival. Mbakwa et al. (2016) similarly identified that early antibiotic exposure, particularly to β-lactams, was linked with greater weight and height z-scores during early childhood, suggesting early-life microbial modulation may affect metabolic programming.
The interplay between antibiotic-induced dysbiosis and immune dysfunction may partially explain the observed health outcomes. Neonatal antibiotic exposure has been shown to alter circulating immune markers and increase susceptibility to functional gastrointestinal disorders and wheezing (Oosterloo et al., 2018; Oosterloo et al., 2020; Salvatore et al., 2019). Additionally, prenatal and perinatal exposure to antibiotics can dysregulate immune development, predisposing infants to allergic diseases (Alhasan et al., 2020; Renz & Skevaki, 2021). These effects may be amplified in preterm neonates, whose immature intestinal and immune systems are particularly sensitive to microbial perturbations (Van Belkum et al., 2020).
Contextual factors such as delivery mode, feeding practices, and geographic setting modulate the impact of antibiotics on the microbiome. Studies by Bokulich et al. (2016) and Reyman et al. (2019) highlighted that cesarean section delivery and formula feeding further delay microbiome maturation. In low- and middle-income countries (LMICs), inappropriate antimicrobial use and inadequate diagnostic infrastructure exacerbate the effects of antibiotic exposure (Luchen et al., 2023). These populations often lack microbiome recovery resources, and azithromycin—commonly used for mass drug administration—was strongly linked to long-term resistance selection.
The findings also align with broader trends in Pediatric microbiome research. Huang et al. (2024) and Kumari et al. (2022) describe how early-life exposure to prescribed medications, especially antibiotics, contributes to microbial imbalance, metabolic dysfunction, and systemic inflammation. The leaky gut hypothesis further posits that dysbiosis increases gut permeability, allowing translocation of microbial products that can disrupt neuroimmune circuits and endocrine function. This mechanism may partially explain the associations between early antibiotic use and neurodevelopmental disorders such as ADHD.
While the reviewed studies vary in methodology, they consistently demonstrate that early antibiotic exposure leads to measurable and often lasting biological effects. The use of next-generation sequencing and metagenomic analyses in studies like those by Gasparrini et al. (2019) and Bargheet et al. (2025) enables a high-resolution view of microbial dynamics and ARG distribution. These advances provide compelling evidence that both microbial diversity and resistome profiles are significantly and chronically impacted by antibiotic exposure in infancy.
From a translational standpoint, these findings underscore the need for more judicious antibiotic prescribing in early life and improved diagnostics to distinguish bacterial from viral infections. Szajewska (2025) suggests microbiota-directed therapies—such as probiotics and prebiotics—could help mitigate microbiome damage, though more trials are needed. Elsharif Bazie et al. (2023) also stress the importance of functional interventions in pediatric gut health, especially for children with antibiotic-associated disorders.
In summary, this review reveals a converging consensus that early-life antibiotic exposure disrupts gut microbial ecology, expands the resistome, and increases risk for chronic diseases. These findings emphasize the need for targeted public health strategies, particularly in LMICs, to regulate antibiotic access and support microbiome recovery. Moving forward, longitudinal studies that track microbiome, resistome, and developmental outcomes into adolescence are needed to fully understand the long-term consequences of early antibiotic use and inform evidence-based pediatric care.
Conclusion
This systematic review provides robust evidence that early-life antibiotic exposure adversely impacts gut microbiome development, contributes to persistent amplification of antimicrobial resistance genes, and increases susceptibility to metabolic, allergic, and neurodevelopmental disorders in children. These findings have implications for pediatric health, particularly as microbiome alterations appear to persist for months or even years post-treatment and can affect long-term immune and metabolic programming.
The data support a call to action for more targeted antibiotic stewardship, particularly in infancy, and emphasize the need for safer therapeutic alternatives and preventive strategies. Policymakers and clinicians must prioritize precision diagnostics, reduce unnecessary prescriptions, and invest in microbiome-supportive interventions, especially in low-resource settings where the risks of overuse and under-regulation are most acute.
Limitations
This review has several limitations. First, although it included a wide range of study designs, the heterogeneity in methods (e.g., microbiome sequencing techniques, antibiotic types, and timing of follow-up) limited direct comparability and precluded meta-analysis. Second, most studies were observational, and while many adjusted for confounders, residual bias cannot be ruled out. Third, variations in antibiotic classes and dosages were not uniformly reported across studies, limiting assessment of dose-response relationships. Finally, the long-term clinical consequences of early microbiome disruption remain understudied beyond early childhood.
References
Alhasan, M. M., Cait, A. M., Heimesaat, M. M., Blaut, M., Klopfeisch, R., & Wedel, A., et al. (2020). Antibiotic use during pregnancy increases offspring asthma severity in a dose-dependent manner. Allergy, 75(8), 1979–1990. https://doi.org/10.1111/all.14297
Aversa, Z., Atkinson, E. J., Schafer, M. J., Theiler, R. N., Rocca, W. A., Blaser, M. J., & LeBrasseur, N. K. (2021). Association of infant antibiotic exposure with childhood health outcomes. Mayo Clinic Proceedings, 96(1), 66–77. https://doi.org/10.1016/j.mayocp.2020.05.021
Bargheet, A., Noordzij, H. T., Ponsero, A. J., Jian, C., Korpela, K., Valles-Colomer, M., ... & Pettersen, V. K. (2025). Dynamics of gut resistome and mobilome in early life: A meta-analysis. EBioMedicine, 114, 105630. https://doi.org/10.1016/j.ebiom.2025.105630
Bokulich, N. A., Chung, J., Battaglia, T., Henderson, N., Jay, M., Li, H., & Blaser, M. J. (2016). Antibiotics, birth mode, and diet shape microbiome maturation during early life. Science Translational Medicine, 8(343), 343ra82. https://doi.org/10.1126/scitranslmed.aad7121
Elsharif, A. B., Al Omar, L., ALHausa, M. A., et al. (2023). Probiotics and functional constipation in children. Journal of Medical – Clinical Research & Reviews, 7(6), 1–6.
Gasparrini, A. J., Wang, B., Sun, X., Kennedy, E. A., Hernandez-Leyva, A., Ndao, M. I., et al. (2019). Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nature Microbiology, 4, 2285–2297. https://doi.org/10.1038/s41564-019-0550-2
Huang, H., Jiang, J., Wang, X., Jiang, K., & Cao, H. (2024). Exposure to prescribed medication in early life and impacts on gut microbiota and disease development. EClinicalMedicine, 68, 102318. https://doi.org/10.1016/j.eclinm.2024.102318
Kumari, R., Yadav, Y., Misra, R., Das, U., Das Adhikari, U., Malakar, P., & Dubey, G. P. (2022). Emerging frontiers of antibiotics use and their impacts on the human gut microbiome. Microbiological Research, 263, 127127. https://doi.org/10.1016/j.micres.2022.127127
Lebeaux, R. M., Karalis, D. B., Lee, J., Whitehouse, H. C., Madan, J. C., Karagas, M. R., & Hoen, A. G. (2022). The association between early life antibiotic exposure and the gut resistome of young children: A systematic review. Gut Microbes, 14(1), 2120743. https://doi.org/10.1080/19490976.2022.2120743
Letouzey, M., Lorthe, E., Marchand-Martin, L., Kayem, G., Charlier, C., Butin, M., ... & Torchin, H. (2022). Early antibiotic exposure and adverse outcomes in very preterm infants at low risk of early-onset sepsis: The EPIPAGE-2 cohort study. The Journal of Pediatrics, 243, 91–98. https://doi.org/10.1016/j.jpeds.2021.10.023
Li, X., Brejnrod, A., Thorsen, J., Zachariasen, T., Trivedi, U., Russel, J., ... & Sørensen, S. J. (2023). Differential responses of the gut microbiome and resistome to antibiotic exposures in infants and adults. Nature Communications, 14(1), 8526. https://doi.org/10.1038/s41467-023-40350-1
Luchen, C. C., Chibuye, M., Spijker, R., Simuyandi, M., Chisenga, C., Bosomprah, S., ... & Harris, V. C. (2023). Impact of antibiotics on gut microbiome composition and resistome in the first years of life in low-to middle-income countries: A systematic review. PLOS Medicine, 20(6), e1004235. https://doi.org/10.1371/journal.pmed.1004235
Mbakwa, C. A., Scheres, L., Penders, J., Mommers, M., Thijs, C., & Arts, I. C. (2016). Early life antibiotic exposure and weight development in children. The Journal of Pediatrics, 176, 105–113. https://doi.org/10.1016/j.jpeds.2016.05.027
McDonnell, L., Gilkes, A., Ashworth, M., Rowland, V., Harries, T. H., Armstrong, D., & White, P. (2021). Association between antibiotics and gut microbiome dysbiosis in children: Systematic review and meta-analysis. Gut Microbes, 13(1), 1870402. https://doi.org/10.1080/19490976.2021.1870402
Oosterloo, B. C., Van Elburg, R. M., Van’t Land, B., Sprikkelman, A. B., & Van der Ent, C. K. (2018). Wheezing and infantile colic are associated with neonatal antibiotic treatment. Pediatric Allergy and Immunology, 29(2), 151–158. https://doi.org/10.1111/pai.12847
Oosterloo, B. C., Van’t Land, B., Knol, E. F., Sprikkelman, A. B., & Van der Ent, C. K. (2020). Neonatal antibiotic treatment is associated with an altered circulating immune marker profile at 1 year of age. Frontiers in Immunology, 10, 2939. https://doi.org/10.3389/fimmu.2019.02939
Patangia, D. V., Grimaud, G., O’Shea, C. A., Ryan, C. A., Dempsey, E., Stanton, C., & Ross, R. P. (2024). Early life exposure of infants to benzylpenicillin and gentamicin is associated with a persistent amplification of the gut resistome. Microbiome, 12(1), 19. https://doi.org/10.1186/s40168-023-01732-6
Princisval, L., Rebelo, F., Williams, B. L., Coimbra, A. C., Crovesy, L., Ferreira, A. L., et al. (2021). Association between the mode of delivery and infant gut microbiota composition up to 6 months of age: A systematic literature review considering the role of breastfeeding. Nutrition Reviews, 80(2), 113–127. https://doi.org/10.1093/nutrit/nuab008
Qi, Q., Wang, L., Gebremedhin, M. A., Liu, X., Li, S., Zhang, H., ... & Wang, Y. (2022). The impact of early-life antibiotics and probiotics on gut microbial ecology and infant health outcomes: A Pregnancy and Birth Cohort in Northwest China (PBCC) study protocol. BMC Pediatrics, 22, 738. https://doi.org/10.1186/s12887-022-03811-3
Reyman, M., Van Houten, M. A., Watson, R. L., Chu, M. L. J., Arp, K., De Waal, W. J., ... & Bogaert, D. (2022). Effects of early-life antibiotics on the developing infant gut microbiome and resistome: A randomized trial. Nature Communications, 13(1), 893. https://doi.org/10.1038/s41467-022-28582-4
Renz, H., & Skevaki, C. (2021). Early life microbial exposures and allergy risks: Opportunities for prevention. Nature Reviews Immunology, 21, 177–191. https://doi.org/10.1038/s41577-020-00426-022.
Salvatore, S., Baldassarre, M. E., Di Mauro, A., Laforgia, N., & Staiano, A. (2019). Neonatal antibiotics and prematurity are associated with an increased risk of functional gastrointestinal disorders in the first year of life. Journal of Pediatrics, 212, 44–51. https://doi.org/10.1016/j.jpeds.2019.05.027
Stuivenberg, G. A., Burton, J. P., Bron, P. A., & Reid, G. (2022). Why are Bifidobacteria important for infants? Microorganisms, 10(2), 278. https://doi.org/10.3390/microorganisms10020278
Szajewska, H. (2025). An overview of early-life gut microbiota modulation strategies. Annals of Nutrition and Metabolism, 81(Suppl 1), 28–33. https://doi.org/10.1159/000541492
Van Belkum, M., Mendoza Alvarez, L., & Neu, J. (2020). Preterm neonatal immunology at the intestinal interface. Cellular and Molecular Life Sciences, 77, 1209–1227. https://doi.org/10.1007/s00018-019-03260-0
Wurm, J., Curtis, N., & Zimmermann, P. (2024). The effect of antibiotics on the intestinal microbiota in children—a systematic review. Frontiers in Allergy, 5, 1458688. https://doi.org/10.3389/falgy.2024.1458688