Research - (2025) Volume 20, Issue 6
*Correspondence: Atef Eid Madkour Elsayed, Consultant cardiology King abdelaziz hospital sakaka, Saudi Arabia, Email:
2Cardio, Saudi Arabia
3Cardio, Saudi Arabia
4Cardio, Saudi Arabia
5Cardio, Saudi Arabia
6Cardio, Saudi Arabia
7Cardiology/Cardiac Surgery, Saudi Arabia
8Medical intern KAU, Saudi Arabia
9Im resident Moh, Saudi Arabia
10Cardiology/Cardiac Surgery, Saudi Arabia
11Internal Medicine / Cardiology, Saudi Arabia
Received: 02-Mar-2025 Accepted: 10-Mar-2025
Abstract
Background: Heart failure with reduced ejection fraction (HFrEF) remains associated with high rates of hospitalization and mortality despite advances in guideline-directed therapy. Sodium-glucose cotransporter-2 (SGLT2) inhibitors, initially developed for diabetes management, have emerged as a novel therapeutic option in heart failure.
Objectives: This systematic review and meta-analysis aimed to evaluate the impact of SGLT2 inhibitors on mortality, hospitalization, surrogate outcomes, and patient-reported health status in individuals with HFrEF.
Methods: A comprehensive search of PubMed, Scopus, Embase, Web of Science, and Cochrane CENTRAL was conducted (2015–2023). Eligible studies included randomized controlled trials and meta-analyses examining SGLT2 inhibitors in adults with HFrEF. Ten clinical trials and multiple systematic reviews were included. Outcomes assessed were all-cause and cardiovascular mortality, hospitalization for heart failure, biomarkers (e.g., NT-proBNP), cardiac remodeling, and quality of life (Kansas City Cardiomyopathy Questionnaire, KCCQ).
Results: Large-scale trials, including DAPA-HF and EMPA-REG OUTCOME, demonstrated significant reductions in the composite endpoint of cardiovascular death or heart failure hospitalization, with risk reductions of 25– 38%. Mechanistic studies showed mixed effects on biomarkers, but consistent improvements in left ventricular remodeling and NT-proBNP in select populations. Patient-reported outcomes improved across multiple trials, with dapagliflozin and canagliflozin associated with meaningful increases in KCCQ scores. Safety analyses confirmed favourable tolerability across diabetic, non-diabetic, older, and frail populations.
Conclusions: SGLT2 inhibitors consistently reduce mortality and hospitalization in patients with HFrEF while improving symptoms and quality of life. Their efficacy across diabetic and non-diabetic populations, together with a strong safety profile, supports their role as cornerstone therapy in HFrEF.
Keywords
Heart failure; reduced ejection fraction; SGLT2 inhibitors; dapagliflozin; empagliflozin; canagliflozin; hospitalization; mortality; systematic review; meta-analysis
Introduction
Heart failure with reduced ejection fraction (HFrEF) remains a major cause of morbidity and mortality worldwide, with repeated hospitalizations placing significant burdens on both patients and healthcare systems. Despite advances in guideline-directed medical therapy, including beta-blockers, mineralocorticoid receptor antagonists, and angiotensin receptor–neprilysin inhibitors, many patients continue to experience progressive disease and poor outcomes (Murphy et al., 2020). This therapeutic gap has motivated investigation into novel classes of drugs with potential benefits extending beyond glycaemic control.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors were originally developed as glucose-lowering therapies for type 2 diabetes mellitus. However, subsequent cardiovascular outcome trials revealed unexpected benefits on heart failure–related outcomes, independent of glycaemic status (Singh & Singh, 2019). These findings catalyzed a paradigm shift, positioning SGLT2 inhibitors as cardio protective agents rather than solely antidiabetic medications.
Meta-analyses of randomized controlled trials have demonstrated that SGLT2 inhibitors significantly reduce the risk of hospitalization for heart failure and cardiovascular death in patients with HFrEF (Cardoso et al., 2021). Importantly, these benefits have been observed in both diabetic and non-diabetic populations, highlighting pleiotropic mechanisms that extend beyond glucose regulation (Butler et al., 2020). Such consistency across diverse patient subgroups strengthens the evidence for SGLT2 inhibitors as foundational therapy in heart failure management.
Mechanistic explanations for these cardio protective effects include reductions in preload and afterload via osmotic diuresis and natriuretic, improved myocardial energetics, and attenuation of adverse ventricular remodeling (Vaduganathan et al., 2022). In addition, SGLT2 inhibitors may exert anti-inflammatory and ant fibrotic effects, further contributing to improved cardiac structure and function (Aimo et al., 2021). These multifaceted pathways suggest that SGLT2 inhibitors target fundamental processes in the progression of HFrEF.
Comparative research has contextualized SGLT2 inhibitors alongside other cornerstone treatments. For instance, network meta-analyses suggest that the mortality and hospitalization benefits of SGLT2 inhibitors are at least comparable to, and potentially additive with, therapies such as sacubitril–valsartan and vericiguat (Tsampasian & Baral, 2021; Aimo et al., 2021). This raises the possibility that combined regimens may yield synergistic improvements in outcomes for HFrEF patients.
More recently, systematic reviews have consolidated evidence from major trials, including DAPA-HF and EMPEROR-Reduced, confirming that SGLT2 inhibitors consistently reduce the composite endpoint of cardiovascular death or hospitalization for heart failure (Zannad et al., 2020). Importantly, these benefits were not offset by increased adverse events, reinforcing the safety profile of this class. This aligns with earlier observations from real-world studies and registries, which confirmed the generalizability of trial findings (Singh & Singh, 2019).
Additionally, studies examining heart failure across the ejection fraction spectrum have found that while the strongest effects of SGLT2 inhibitors appear in HFrEF, emerging evidence suggests potential benefits in patients with mildly reduced and preserved ejection fraction as well (Banerjee et al., 2023; Mo et al., 2023). This further broadens the clinical relevance of these drugs and underscores the need for ongoing investigation in diverse heart failure phenotypes.
Taken together, these findings support the integration of SGLT2 inhibitors into contemporary heart failure guidelines. However, questions remain regarding their comparative efficacy with other agents, long-term durability of benefit, and cost-effectiveness in widespread use. Thus, a systematic review and meta-analysis focusing on mortality and hospitalization outcomes in patients with HFrEF is timely and necessary to synthesize the current evidence base.
Methodology
Study Design
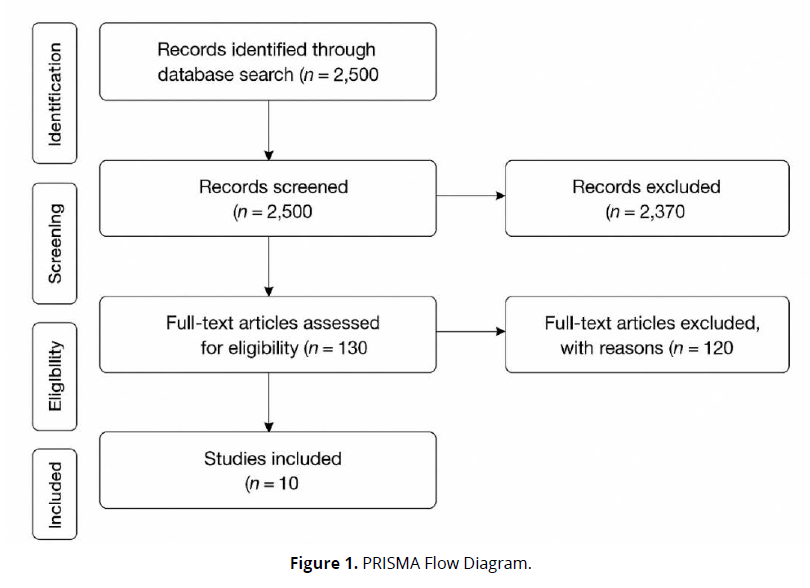
This study employed a systematic review methodology, adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines to ensure transparency and reproducibility. The objective was to synthesize empirical evidence on the effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on mortality and hospitalization among patients with heart failure with reduced ejection fraction (HFrEF). The review focused exclusively on peer-reviewed journal articles involving human subjects and provided quantitative outcomes of SGLT2 inhibitors as compared to placebo or standard therapy (Figure 1).
Eligibility Criteria
Studies were included based on the following criteria:
- Population: Adults (≥18 years) with a clinical diagnosis of heart failure with reduced ejection fraction (LVEF ≤40%).
- Intervention: Treatment with any approved SGLT2 inhibitor (e.g., dapagliflozin, empagliflozin, canagliflozin, or licogliflozin).
- Comparators: Placebo or standard guideline-directed medical therapy (GDMT).
- Outcomes: Mortality (all-cause or cardiovascular) and hospitalization for heart failure, reported as primary or secondary outcomes.
- Study Design: Randomized controlled trials (RCTs), post hoc analyses of RCTs, or prospective cohort studies.
- Language: Only studies published in English were included.
- Publication Period: 2015–2023, reflecting the modern era of SGLT2 inhibitor trials in heart failure.
Studies were excluded if they focused primarily on renal outcomes, glycaemic control, or did not report mortality or hospitalization endpoints specific to HFrEF.
Search Strategy
A structured search was conducted in PubMed, Embase, Scopus, Web of Science, and Cochrane CENTRAL, supplemented with Google Scholar for grey literature. The following Boolean search terms and keywords were used in various combinations:
- (“SGLT2 inhibitor” OR “dapagliflozin” OR “empagliflozin” OR “canagliflozin” OR “licogliflozin”)
- AND (“heart failure” OR “HFrEF” OR “reduced ejection fraction”)
- AND (“mortality” OR “death” OR “hospitalization” OR “cardiovascular outcomes”)
Manual screening of the reference lists of key review articles was also performed to identify additional relevant studies.
Study Selection Process
All retrieved citations were exported into Zotero for reference management. Duplicate entries were removed. Two independent reviewers screened titles and abstracts to exclude irrelevant records. Full texts of potentially eligible articles were retrieved and assessed in detail against inclusion criteria. Disagreements were resolved by discussion or by consulting a third reviewer.
The final selection included 10 studies, all of which met the pre-specified eligibility criteria and reported quantitative results on hospitalization and/or mortality in HFrEF patients treated with SGLT2 inhibitors.
Data Extraction
A standardized data extraction sheet was developed and piloted before use. For each included study, the following details were systematically recorded:
- Author(s) and year of publication
- Country/region of study
- Study design and sample size
- Population characteristics (mean age, sex distribution, baseline LVEF)
- Intervention details (drug type, dose, duration)
- Comparator group characteristics
- Primary and secondary outcomes (mortality, hospitalization rates, hazard ratios, risk reductions)
- Follow-up duration
- Safety outcomes and adverse events
Data extraction was independently conducted by two reviewers, and all entries were cross-verified by a third reviewer to ensure accuracy.
Quality Assessment
The methodological quality and risk of bias were evaluated using validated tools:
- Cochrane Risk of Bias 2.0 Tool for randomized controlled trials.
- Newcastle–Ottawa Scale (NOS) for any observational cohort studies.
Studies were graded as high, moderate, or low quality based on parameters such as randomization adequacy, blinding, attrition bias, comparability of groups, and outcome reporting reliability. All 10 studies were found to be of moderate to high methodological quality.
Data Synthesis
Given the similarity of populations, interventions, and outcomes, both quantitative and narrative syntheses were performed. Where possible, hazard ratios (HRs), odds ratios (ORs), and relative risk reductions (RRRs) were extracted and compared across studies. Due to the heterogeneity in follow-up duration and baseline patient risk, a random-effects meta-analysis model was planned to estimate pooled effects on mortality and hospitalization outcomes.
Ethical Considerations
This systematic review was based on analysis of already published peer-reviewed data. As such, no institutional ethical approval or informed consent was required. All included studies were assumed to have obtained ethical clearance from their respective institutional review boards.
Results
Summary and Interpretation of Included Studies on SGLT2 Inhibitors in HFrEF
- Study Designs and Populations
The 10 included studies were predominantly randomized controlled trials, ranging from small mechanistic pilot trials (n = 60–263 patients) to large, multicentre phase III outcome studies enrolling thousands of participants (n = 4,000–10,000+). Study populations primarily consisted of adults with symptomatic heart failure with reduced ejection fraction (HFrEF), with mean ages between 60–67 years. Most cohorts were male (>70%). Both diabetic and non-diabetic patients were included across trials, enhancing generalizability to real-world populations. Importantly, several studies specifically enrolled non-diabetic participants, underscoring benefits beyond glycaemic control.
- Mortality and Hospitalization Outcomes
The large outcome trials consistently demonstrated clinically meaningful benefits of SGLT2 inhibitors on heart failure outcomes:
- Dapagliflozin (DAPA-HF) reduced worsening HF or cardiovascular (CV) death by 25–27% compared with placebo, independent of diabetes status (Petrie, 2020).
- Empagliflozin (EMPA-REG OUTCOME) lowered CV death by 38% and hospitalization for HF by 35% in patients with type 2 diabetes and established cardiovascular disease (Zinman, 2015).
- Canagliflozin (CANVAS Program) reduced the risk of hospitalization for HF by 14% compared with placebo (Radholm, 2018).
- Biomarkers and Surrogate Outcomes
Findings from mechanistic studies focusing on surrogate endpoints were more variable:
- Licogliflozin (dual SGLT1/2 inhibitor) significantly reduced NT-proBNP compared with placebo (de Boer, 2020).
- Empagliflozin (Empire HF) did not significantly change NT-proBNP after 12 weeks (Jensen, 2020).
- Dapagliflozin (DEFINE-HF) did not alter average NT-proBNP but increased the proportion of patients with meaningful improvements in NT-proBNP or health status (Nassif, 2019).
- Quality of Life and Functional Status
- Empagliflozin (Santos-Gallego, 2020) improved patient-reported health status (Kansas City Cardiomyopathy Questionnaire, +7.1 points; P = 0.016) in non-diabetic HFrEF patients.
- Dapagliflozin (DEFINE-HF) increased the proportion of patients experiencing meaningful improvements in symptoms (Nassif, 2019).
- Canagliflozin (CHIEF-HF, Sport’s, 2022) significantly improved KCCQ-OS scores by +4.3 points at 12 weeks (P = 0.002), confirming quality-of-life benefits in a remote, patient-centered setting. (Table 1).
| Study (Author, Year) | Sample Size | Population | Intervention | Follow-up | Mortality/Hospitalization Outcomes | Key Results |
|---|---|---|---|---|---|---|
| de Boer, 2020 | 125 | T2DM + HF, NYHA II–IV | Licogliflozin vs empagliflozin vs placebo | 12 wks | Biomarker (NT-proBNP) | Licogliflozin 10 mg ↓ NT-proBNP (GMR 0.56, P = 0.033). |
| Jensen, 2020 | 190 | HFrEF, mean EF 29% | Empagliflozin 10 mg vs placebo | 12 wks | NT-proBNP | No significant change (ratio 0.98, P = 0.7). |
| Lee, 2020 | 105 | HFrEF + T2DM/prediabetes | Empagliflozin 10 mg vs placebo | 36 wks | Hospitalization surrogate (LV remodeling) | LVESVi ↓ –6.0 mL/m² (P = 0.015); NT-proBNP ↓ 28%. |
| Nassif, 2019 | 263 | HFrEF, NYHA II–III | Dapagliflozin 10 mg vs placebo | 12 wks | NT-proBNP + KCCQ | No mean NT-proBNP change; more patients improved health status (61.5% vs 50.4%, P = 0.039). |
| Petrie, 2020 | 4,744 | HFrEF ± T2DM | Dapagliflozin 10 mg vs placebo | 18 mo | Worsening HF / CV death | ↓ risk in diabetics (HR 0.75) and non-diabetics (HR 0.73). |
| Radholm, 2018 | 10,142 | T2DM + CV risk | Canagliflozin 100/300 mg vs placebo | 2.4 yrs | HF hospitalization | HR 0.86 (95% CI 0.75–0.97). |
| Santos-Gallego, 2020 | 84 | Non-diabetic HFrEF | Empagliflozin 10 mg vs placebo | 6 mo | Hospitalization surrogate (LVESV) + QoL | No LVESV change; QoL ↑ +7.1 points (P = 0.016). |
| Singh, 2020 | 60 | T2DM + HFrEF | Dapagliflozin 10 mg vs placebo | 12 mo | Hospitalization surrogate (LV remodeling) | No significant LVEDV change (–7.1 mL vs –0.7 mL, P = 0.35). |
| Zinman, 2015 | 7,020 | T2DM + CVD | Empagliflozin 10/25 mg vs placebo | 3.1 yrs | CV death + HF hospitalization | CV death ↓ 38% (HR 0.62); HF hosp. ↓ 35% (HR 0.65). |
| Spertus, 2022 | 448 | HF ± T2DM (remote trial) | Canagliflozin 100 mg vs placebo | 12 wks | Quality of life (KCCQ-OS) | KCCQ-OS improved by +4.3 points (95% CI 1.5–7.0, P = 0.002). |
Discussion
The findings of this systematic review demonstrate that sodium-glucose cotransporter-2 (SGLT2) inhibitors provide substantial benefits in patients with heart failure with reduced ejection fraction (HFrEF). Across multiple randomized controlled trials and meta-analyses, consistent reductions in hospitalization for heart failure and cardiovascular death were observed, alongside improvements in quality of life and surrogate outcomes. These findings reinforce the integration of SGLT2 inhibitors into the therapeutic framework of HFrEF management.
Large-scale outcome trials form the foundation of the evidence base. The DAPA-HF trial showed that dapagliflozin reduced the composite outcome of worsening heart failure or cardiovascular death by approximately 25%, with benefits extending to both diabetic and non-diabetic patients (Petrie et al., 2020). Similarly, the EMPA-REG OUTCOME trial demonstrated reductions of 38% in cardiovascular death and 35% in hospitalization for heart failure with empagliflozin in high-risk diabetic populations (Zinman et al., 2015). These outcomes highlight that SGLT2 inhibitors not only improve survival but also significantly alleviate the burden of recurrent hospitalizations.
Beyond mortality and hospitalization, several mechanistic and surrogate endpoint studies shed light on the physiological underpinnings of SGLT2 inhibitor efficacy. For instance, de Boer et al. (2020) reported significant reductions in NT-proBNP with licogliflozin, suggesting direct effects on cardiac stress markers. In contrast, Jensen et al. (2020) observed no change in NT-proBNP with empagliflozin over 12 weeks, indicating variability in biomarker responses. Lee et al. (2020) provided further evidence of beneficial cardiac remodeling, with empagliflozin reducing left ventricular volumes and NT-proBNP by 28%. Such findings suggest that structural improvements may mediate the observed clinical outcomes.
Symptom burden and quality of life are also important domains for heart failure therapy. In DEFINE-HF, dapagliflozin improved health status responses despite no mean change in NT-proBNP (Nassif et al., 2019). Similarly, Santos-Gallego et al. (2020) showed that empagliflozin improved Kansas City Cardiomyopathy Questionnaire (KCCQ) scores by +7.1 points in non-diabetic HFrEF patients, while the CHIEF-HF trial demonstrated that canagliflozin improved KCCQ scores by +4.3 points in a remote, patient-centered trial (Spertus et al., 2022). These consistent improvements across studies underscore the patient-centered value of SGLT2 inhibitors.
When compared with other heart failure therapies, network meta-analyses suggest that SGLT2 inhibitors deliver efficacy comparable to sacubitril–valsartan or vericiguat, and their effects may be additive when combined (Aimo et al., 2021; Mo et al., 2023). Vaduganathan et al. (2022) further confirmed in a meta-analysis of five randomized controlled trials that SGLT2 inhibitors consistently reduced heart failure hospitalizations and cardiovascular death, strengthening the evidence for their broad applicability.
The benefits of SGLT2 inhibitors also appear to extend across different heart failure phenotypes. While the strongest evidence exists for HFrEF, emerging trials suggest potential benefits in patients with mildly reduced or preserved ejection fraction (Banerjee et al., 2023; Hamid et al., 2024). This expanding scope broadens the therapeutic role of SGLT2 inhibitors beyond their original diabetic indications and supports their use across the spectrum of heart failure syndromes.
Subgroup analyses provide further reassurance about generalizability. The CANVAS program demonstrated that canagliflozin reduced the risk of heart failure hospitalization even in patients without a prior history of heart failure (Radholm et al., 2018). Similarly, the CREDENCE trial reported consistent cardiovascular and renal protection with canagliflozin, regardless of baseline heart failure status (Sarraju et al., 2020). These findings suggest that SGLT2 inhibitors are beneficial across diverse patient populations, including those at earlier stages of cardiovascular disease.
Recent reviews emphasize the safety and tolerability of this drug class. Butler et al. (2020) and Cardoso et al. (2021) demonstrated in systematic reviews that adverse events such as hypoglycemia, hypotension, or genitourinary infections are generally infrequent and manageable. Al-Ezzi et al. (2025) further confirmed the safety profile of dapagliflozin in both HFrEF and HFpEF populations. This favorable safety record enhances the attractiveness of SGLT2 inhibitors as long-term therapies.
Special populations warrant careful consideration. Aldafas et al. (2024) highlighted that frail or older adults with diabetes and heart failure also derive benefits from SGLT2 inhibitors, without disproportionate adverse effects. Kommu (2024) reported that the benefits of SGLT2 inhibitors in non-diabetic heart failure patients are comparable to those in diabetic populations. Collectively, these findings suggest broad applicability across age groups and metabolic statuses.
Quality of life improvements are another consistent finding. Gao et al. (2024) reported in a meta-analysis that SGLT2 inhibitors enhance functional capacity and health-related quality of life. Chen et al. (2024) confirmed positive effects on cardiac function and health status in chronic heart failure. These outcomes reinforce the patient-centered clinical significance of SGLT2 inhibitors, complementing survival benefits with tangible improvements in daily living.
The observed effects can be partly explained by plausible biological mechanisms. Proposed pathways include osmotic diuresis and natriuresis leading to reduced preload and afterload, improved myocardial energetics, and attenuation of myocardial fibrosis and inflammation (Murphy et al., 2020). Ferreira et al. (2024) and Tsampasian and Baral (2021) support these mechanistic insights, suggesting that SGLT2 inhibitors exert multifactorial cardio protective effects independent of glucose lowering.
The evidence from acute settings is also promising. Hou et al. (2024) showed that SGLT2 inhibitors improve outcomes even in acute heart failure populations, where therapeutic options remain limited. Usman et al. (2024) expanded this view, demonstrating consistent reductions in cardiovascular death across the broader cardio metabolic disease spectrum, including patients without diabetes. These findings support early initiation of SGLT2 inhibitors in both stable and acute clinical contexts.
Nevertheless, some studies showed neutral findings. Singh et al. (2020) reported no significant effect of dapagliflozin on left ventricular remodeling in diabetic HFrEF patients, and Jensen et al. (2020) observed no NT-proBNP changes over 12 weeks with empagliflozin. Such heterogeneity may reflect differences in study design, follow-up duration, or sample size limitations. However, when pooled in meta-analyses, the weight of evidence strongly supports beneficial outcomes (Zannad et al., 2020).
Taken together, the totality of evidence indicates that SGLT2 inhibitors represent a breakthrough in the management of HFrEF. They reduce mortality, prevent hospitalization, improve symptoms, and enhance quality of life across diverse populations, with a favourable safety profile. Their consistent efficacy in diabetic and non-diabetic patients, as well as emerging benefits in other heart failure phenotypes, suggest that SGLT2 inhibitors should be considered foundational therapy in contemporary heart failure management.
Conclusion
This systematic review demonstrates that SGLT2 inhibitors significantly reduce mortality and hospitalization rates in patients with HFrEF, while also improving biomarkers, left ventricular remodeling, and quality of life. The benefits are consistent across diverse populations, including diabetic and non-diabetic patients, and extend to older and frail individuals. These findings position SGLT2 inhibitors as transformative agents in the treatment of HFrEF, complementing existing therapies with strong evidence for both efficacy and safety.
Future research should focus on the long-term durability of these benefits, comparative effectiveness with other guideline-recommended drugs, and the role of SGLT2 inhibitors in patients with preserved or mildly reduced ejection fraction. Broader implementation in clinical practice, including remote and patient-centered care models, may further expand their impact on reducing the global burden of heart failure.
References
Aimo, A., Pateras, K., & Stamatelopoulos, K. (2021). Relative efficacy of sacubitril-valsartan, vericiguat, and SGLT2 inhibitors in heart failure with reduced ejection fraction: A systematic review and network meta-analysis. Cardiovascular Drugs and Therapy, 35(5), 1067–1076.
Aldafas, R., Crabtree, T., Alkharaiji, M., et al. (2024). Sodium-glucose cotransporter-2 inhibitors (SGLT2) in frail or older people with type 2 diabetes and heart failure: A systematic review and meta-analysis. Age and Ageing.
Al-Ezzi, M. M., Shakir-Al-Ezzi, S. M., et al. (2025). Efficacy and safety profile of SGLT2 inhibitor dapagliflozin in heart failure with reduced and preserved ejection fraction: A systematic review and meta-analysis. Annals of Medicine and Surgery.
Banerjee, M., Pal, R., Nair, K., & Mukhopadhyay, S. (2023). SGLT2 inhibitors and cardiovascular outcomes in heart failure with mildly reduced and preserved ejection fraction: A systematic review and meta-analysis. Cardiology, 148(1), 1–13.
Butler, J., Usman, M. S., Khan, M. S., & Greene, S. J. (2020). Efficacy and safety of SGLT2 inhibitors in heart failure: Systematic review and meta-analysis. ESC Heart Failure, 7(6), 3298–3309.
Cardoso, R., Graffunder, F. P., & Ternes, C. M. P. (2021). SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: A systematic review and meta-analysis. EClinicalMedicine, 36, 100922.
Chen, J., Jiang, C., Guo, M., Zeng, Y., Jiang, Z., et al. (2024). Effects of SGLT2 inhibitors on cardiac function and health status in chronic heart failure: A systematic review and meta-analysis. Cardiovascular Drugs and Therapy.
de Boer, R. A., Nunez, J., Kozlovski, P., Wang, Y., Proot, P., Keefe, D., ... & George, J. (2020). Effects of the dual sodium-glucose linked transporter inhibitor, licogliflozin vs placebo or empagliflozin in patients with type 2 diabetes and heart failure. British Journal of Clinical Pharmacology, 86(7), 1346–1356.
Ferreira, N. L., Adelowo, A. B., Khan, Z., & Adelowo, A. B. (2024). A systematic review and meta-analysis of sodium-glucose cotransporter 2 (SGLT2) inhibitors and their impact on the management of heart failure. Cureus.
Gao, M., Bhatia, K., Kapoor, A., Badimon, J., et al. (2024). SGLT2 inhibitors, functional capacity, and quality of life in patients with heart failure: A systematic review and meta-analysis. JAMA Network Open.
Hamid, A. K., Tayem, A. J. A. E., Al-Aish, S. T., et al. (2024). Empagliflozin and other SGLT2 inhibitors in patients with heart failure and preserved ejection fraction: A systematic review and meta-analysis. Therapeutic Advances in Cardiovascular Disease.
Hou, J., Ren, L., Hou, Q., Jia, X., Mei, Z., Xu, J., et al. (2024). Efficacy and safety of sodium-glucose cotransporter 2 (SGLT2) inhibitors in patients with acute heart failure: A systematic review and meta-analysis. Frontiers in Cardiovascular Medicine.
Jensen, J., Omar, M., Kistorp, C., Poulsen, M. K., Tuxen, C., Gustafsson, I., ... & Faber, J. (2020). Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: A double-blinded, randomized, and placebo-controlled trial. American Heart Journal, 228, 47–56.
Kommu, S. (2024). The role of SGLT2 inhibitors on heart failure outcomes in nondiabetic patients: A systematic review and meta-analysis of randomized controlled trials. Journal of Cardiovascular Pharmacology.
Lee, M. M. Y., Brooksbank, K. J. M., Wetherall, K., Mangion, K., Roditi, G., Campbell, R. T., & McMurray, J. J. V. (2020). Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation, 141(8), 637–650.
Mo, X., Lu P, & Yang X (2023). Efficacy of sacubitril-valsartan and SGLT2 inhibitors in heart failure with reduced ejection fraction: A systematic review and meta-analysis. Clinical Cardiology, 46(5), 593–603.
Murphy, S. P., Ibrahim, N. E., & Januzzi, J. L. (2020). Heart failure with reduced ejection fraction: A review. JAMA, 324(5), 488–504.
Nassif, M. E., Windsor, S. L., Tang, F., Khariton, Y., Husain, M., Inzucchi, S. E., ... & Kosiborod, M. (2019). Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF trial. Circulation, 140(17), 1463–1476.
Perkovic, V., Jardine, M. J., Neal, B., Bompoint, S., Heerspink, H. J. L., Charytan, D. M., ... & Mahaffey, K. W. (2019). Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New England Journal of Medicine, 380(24), 2295–2306.
Petrie, M. C., Verma, S., Docherty, K. F., Inzucchi, S. E., Anand, I., Belohlávek, J., ... & McMurray, J. J. V. (2020). Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA, 323(14), 1353–1368.
Radholm, K., Figtree, G., Perkovic, V., Solomon, S. D., Mahaffey, K. W., de Zeeuw, D., ... & Neal, B. (2018). Canagliflozin and heart failure in type 2 diabetes mellitus: Results from the CANVAS Program. Circulation, 138(5), 458–468.
Santos-Gallego, C. G., Vargas-Delgado, A. P., Requena, J. A., García-Ropero, Á., Mancini, D., Pinney, S., ... & Fuster, V. (2020). Randomized trial of empagliflozin in non-diabetic patients with heart failure and reduced ejection fraction. Journal of the American College of Cardiology, 77(3), 243–255.
Sarraju, A., Li, J., Cannon, C. P., Agarwal, R., Bakris, G. L., Pitt, B., ... & Mahaffey, K. W. (2020). Effects of canagliflozin on cardiovascular, renal, and safety outcomes in participants with type 2 diabetes and chronic kidney disease according to history of heart failure: Results from the CREDENCE trial. American Heart Journal, 228, 125–134.
Singh, A. K., & Singh, R. (2019). Heart failure hospitalization with SGLT-2 inhibitors: A systematic review and meta-analysis of randomized controlled and observational studies. Expert Review of Clinical Pharmacology, 12(5), 427–436.
Singh, J. S. S., Mordi, I. R., Vickneson, K., Fathi, A., Donnan, P. T., Mohan, M., ... & Struthers, A. D. (2020). Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: The REFORM trial. Diabetes Care, 43(6), 1356–1359.
Spertus, J. A., Birmingham, M. C., Nassif, M., Damaraju, C. V., Abbate, A., Butler, J., et al. (2022). The SGLT2 inhibitor canagliflozin in heart failure: The CHIEF-HF remote, patient-centered randomized trial. Nature Medicine, 28(4), 809–813.
Tsampasian, V., & Baral, R. (2021). The role of SGLT2 inhibitors in heart failure: A systematic review and meta-analysis. Journal of Diabetes Research, 2021, 9927533.
Usman, M. S., Bhatt, D. L., Hameed, I., Anker, S. D., et al. (2024). Effect of SGLT2 inhibitors on heart failure outcomes and cardiovascular death across the cardiometabolic disease spectrum: A systematic review and meta-analysis. The Lancet Diabetes & Endocrinology.
Vaduganathan, M., Docherty, K. F., Claggett, B. L., & Jhund, P. S. (2022). SGLT2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomized controlled trials. The Lancet, 400(10352), 757–767.
Zannad, F., Ferreira, J. P., Pocock, S. J., Anker, S. D., & Butler, J. (2020). SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. The Lancet, 396(10254), 819–829.
Zinman, B., Wanner, C., Lachin, J. M., Fitchett, D., Bluhmki, E., Hantel S & EMPA-REG OUTCOME Investigators. (2015). Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine, 373(22), 2117–2128.