Research Article - (2025) Volume 20, Issue 6
Efficacy Of Different Doses Of Photobiomodulation On Palmo Plantar Erythrodysesthesia Syndrome Associated With Cancer Th
Aya M. M. Mohamed1,2*, Eman M. Othman1, Karim I. Saafan1,4, Amr A. Hafez3, Labib A. Gaber Shami5 and Maha Abd-ELmonem1,6*Correspondence: Aya M. M. Mohamed, Department of Physical Therapy for Surgery, Faculty of Physical Therapy, Cairo University, Egypt, Tel: (+02) 01151582194, Email:
2Department of Physical Therapy for Surgery, Faculty of Physical Therapy, May University at Cairo, Egypt
3Department of Surgical Oncology, National Cancer Institute, Cairo University, Egypt
4Department of Physical Therapy for Surgery, Faculty of Physical Therapy, Horus University-Egypt, New Damietta, Egypt
5Faculty of Physical Therapy, Jazan University, KSA
6Department of Physical Therapy for Surgery, Faculty of Physical Therapy, 6th October University, Egypt
Received: 10-Oct-2025 Published: 24-Nov-2025
Abstract
Background: Hand-Foot Syndrome (HFS) is a common and debilitating side effect of cancer therapies, particularly those involving capecitabine and 5-Fluorouracil (5-FU). HFS manifests as painful redness, swelling, and tingling on the palms and soles, severely impacting patients’ quality of life. Photobiomodulation (PBM), a non-invasive therapy using light to stimulate cellular healing, has been proposed as an effective treatment for HFS. Previous studies have indicated that the intensity of PBM may influence its therapeutic efficacy, with 2 J/ cm² and 4 J/cm² doses showing promise in reducing HFS symptoms.
Purpose: This study aims to compare the efficacy of PBM at two different fluence levels (2 J/cm² and 4 J/ cm²) in reducing the severity of HFS and associated symptoms in cancer patients undergoing chemotherapy. Specifically, it evaluates improvements in pain reduction, functional abilities, and overall symptom burden.
Patients and Methods: This randomized controlled trial included 40 participants (24 females and 16 males) undergoing cancer treatment with capecitabine or 5-FU and diagnosed with palmo-plantar erythrodysesthesia were randomly assigned to two groups (n=20 each). Group A (20 patients) received PBM at 755 nm with a dose of 2 J/cm², while Group B (20 patients) received PBM at 4 J/cm², both three times weekly alongside standard medical therapy. Treatment was applied three times a week for four weeks. Outcomes and Measures: Participants were assessed before and after treatment using the Hand-Foot Syndrome-14 (HFS-14) questionnaire and the Visual Analog Scale (VAS) for pain. Statistical analyses were performed to compare pre- and post-treatment outcomes within and between groups. Results: Statistical analysis revealed significant post-treatment improvements in both groups across all outcome measures: visual analogue scale (VAS), HFS-14 questionnaire scores, and HFS grades. However, Group B demonstrated significantly greater improvements than Group A in VAS reduction (75.13% vs. 45.88%, p<0.0001) and HFS-14 score reduction (88.36% vs. 66.90%, p<0.0001). These findings suggest that higher-dose PBM may offer superior clinical benefits in reducing HFS symptoms in cancer patients.
Conclusion: PBM, especially at 4 J/cm², is an effective non-invasive therapy for managing HFS in cancer patients. The findings suggest that higher fluence levels provide enhanced therapeutic benefits, particularly in reducing pain and improving functional abilities, quality of life, and social participation. PBM should be considered a promising adjunctive treatment in cancer rehabilitation protocols for managing chemotherapy-induced dermatological toxicity.
Keywords
Cancer therapy, Chemotherapy-induced toxicity, Functional mobility, Hand-foot syndrome, Palmo-Plantar Erythrodysesthesia, Photobiomodulation therapy.
Introduction
Hand-foot syndrome (HFS) is a common (The prevalence of HFS ranges from 6% to 45% in patients treated with 5-FU and from 22% to 77% in those receiving capecitabine, with an average of around 50–60%.27,28) and debilitating side effect of certain cancer therapies, including capecitabine and 5-fluorouracil (5-FU), which are widely used in the treatment of various cancers 1. HFS manifests as painful redness, swelling, and tingling on the palms of the hands and soles of the feet, significantly affecting patients' quality of life. This condition can lead to functional impairments, making it difficult for patients to perform daily activities such as walking, driving, or even standing 2. The severity of HFS varies among individuals and is often correlated with the dose and duration of chemotherapy treatments, with patients on infusional fluorouracil or oral capecitabine being particularly vulnerable to its onset 3.
Photobiomodulation (PBM) has emerged as a promising therapeutic option for managing HFS. PBM, commonly delivered through Low-Level Laser Therapy (LLLT), involves the application of light to the skin to promote healing at the cellular level 4. This non-invasive treatment is known to stimulate cellular processes such as collagen synthesis, reduce inflammation, and improve circulation, all of which can alleviate the symptoms of HFS 3. Recent studies have shown that PBM can significantly reduce pain and functional impairments associated with HFS, making it an ideal adjunctive therapy in cancer treatment protocols 2.
The efficacy of PBM in treating HFS depends largely on the intensity and frequency of light application. In previous studies, two commonly used light intensities for PBM treatment are 2 J/cm² and 4 J/cm², both of which have demonstrated effectiveness in reducing HFS severity 3. While lower intensity treatments like 2 J/cm² are often well-tolerated, higher intensities such as 4 J/cm² may offer more pronounced therapeutic effects. However, the optimal treatment parameters for PBM in HFS management remain a subject of ongoing investigation 5.
The present study aims to evaluate the comparative effectiveness of two different PBM intensities-2 J/cm² and 4 J/cm²-in the treatment of HFS in patients undergoing cancer therapy with capecitabine or 5-FU.
Methods
Design of the study
This study was a single blinded Pre-Post-test randomized clinical trial
Ethical Approval
Each patient completed an informed consent sheet after being fully informed of their rights before taking part in the trial. The Institutional Review Board of Cairo University's Faculty of Physical Therapy granted ethical permission (No: P.T.REC/012/005393) prior to the study execution. The Declaration of Helsinki Guidelines for Human Research was followed in the conduct of the current investigation.
Sample size calculation
The calculation of the sample size was conducted using the statistical software G-Power V.3.1 (Universitat Kiel, Germany) employing the Χ2 homogeneity test. Equal sample sizes were allocated to both the groups, assuming an efficacy of 57% for PBM treatment, as previously estimate. The significance level was set at 5%, and the statistical power at 80%, resulting in a sample size of n=40, with a consideration for a 10% potential loss during the study. This ensures adequate power to detect any significant differences between the treatment groups.
Subjects
The study included a total of 40 participants (16 males and 24 females), who were selected based on specific inclusion and exclusion criteria. All participants were patients undergoing cancer therapy, specifically those receiving either oral capecitabine or continuous infusion of 5-fluorouracil (5-FU), both of which are known to cause Hand-Foot Syndrome (HFS). The participants were recruited from the National Cancer Institute, and were randomly assigned to two groups: Group A and Group B, with an equal allocation (1:1 ratio). The inclusion criteria required participants to have a pathological diagnosis of cancer, be ambulant, and have grade one or two hand-foot syndrome as per the HFS-14 questionnaire. The participants also had to be conscious and able to provide informed consent. Exclusion criteria eliminated patients with conditions such as metallic implants, cardiac pacemakers, significant circulatory disorders, a history of skin malignancy, or those who missed more than 3 treatment sessions. All participants provided written consent after receiving a detailed explanation of the treatment and measurement procedures, ensuring ethical compliance and voluntary participation. The study aimed to assess the effectiveness of photobiomodulation therapy (PBM) at two different intensities in alleviating HFS symptoms and improving functional outcomes in these patients.
Measurement equipment and procedures
The study utilized a combination of validated measurement equipment and standardized procedures to assess the severity of Hand-Foot Syndrome (HFS) and the intensity of pain experienced by the participants. The Hand-Foot Syndrome-14 (HFS-14) questionnaire was used to assess the severity and impact of HFS symptoms. This questionnaire consists of 14 items designed to evaluate symptoms such as pain, redness, swelling, tingling, and functional impairments in the palms and soles of the hands and feet. Participants rated the severity of each symptom on a scale from 0 to 3, where 0 indicated no symptoms and 3 indicated severe symptoms. The questionnaire also assessed the frequency of these symptoms and their interference with daily activities like walking, standing, and performing regular tasks.
In addition to the HFS-14 questionnaire, the Visual Analog Scale (VAS) was employed to measure pain intensity. The VAS consists of a 10-centimeter line with endpoints labeled as "no pain" on the left and "worst pain imaginable" on the right. Participants were asked to mark their current pain level along the line, and the distance from the left endpoint to the mark was measured to determine the pain intensity.
Pre-treatment and post-treatment evaluations were performed to assess the impact of PBM therapy on both symptom severity and pain levels. The measurements were taken at baseline and after the completion of the four-week treatment period, allowing for a direct comparison of the effectiveness of the intervention in reducing HFS symptoms and improving functional outcomes.
Therapeutic equipment and procedures
The therapeutic intervention in this study was delivered using a Low-Level Laser Therapy (LLLT) unit, specifically the EME PHYSO (S.N. 9980002\08) device, which emits 755 nm wavelength light. This wavelength was chosen due to its proven efficacy in penetrating the skin and promoting healing at the cellular level. The device used for the Photobiomodulation (PBM) therapy consisted of a light-emitting diode (LED) cluster with five LEDs operating at 250 mW each, arranged in a 20 cm² area. This cluster emits low-level laser light to the treatment area, providing the required energy of either 2 J/cm² or 4 J/cm² depending on the group assignment (Group A or Group B). The treatment was applied directly to the palmoplantar areas (the palms and soles) of the hands and feet, which are common sites of hand-foot syndrome (HFS) in patients undergoing cancer therapies like capecitabine or 5-FU.
The treatment procedure involved applying the laser light to the targeted areas using a contact technique, where the laser probe was held directly against the skin. Each participant received treatment three times per week for four weeks, totalling 12 treatment sessions. In Group A (2 J/cm²), the treatment intensity was set at 2 J/cm², while in Group B (4 J/cm²), the intensity was set at 4 J/cm². The laser therapy aimed to reduce the severity of symptoms associated with HFS, such as pain, redness, swelling, and tingling, and to promote healing of the affected skin by stimulating cellular processes such as collagen production and tissue repair. Prior to treatment, patient skin was cleaned to remove any surface oils or lotions, ensuring effective laser penetration, and protective goggles were provided to shield the eyes from the laser light. The procedure also involved checking for any contraindications to laser therapy, such as photosensitivity or the use of photosensitizing medications, to ensure patient safety.
By comparing the outcomes of the two treatment groups, this study seeks to provide evidence for the optimal intensity of PBM for managing hand-foot syndrome in cancer patients. The findings could inform clinical practice by offering guidelines for PBM usage in this context, potentially improving the quality of life for patients undergoing chemotherapy. Given the widespread use of capecitabine and 5-FU in cancer treatment, understanding the benefits of PBM in managing HFS could have a significant impact on patient care 3.
Results
Statistical analysis
Data were screened, for normality assumption test and homogeneity of variance. Normality test of data using Shapiro-Wilk, that reflect the data was normally distributed (P>0.05) after removal outliers that detected by box and whiskers plots. Additionally, Levene's test for testing the homogeneity of variance revealed that there was no significant difference (P>0.05). All these findings allowed to conducted parametric and non-parametric analysis. The data is normally distributed and parametric analysis is done.
The statistical analysis was conducted by using statistical SPSS Package program version 25 for Windows (SPSS, Inc., Chicago, IL). Quantitative data for patient’s demographic data (age, weight, height, and BMI), VAS and HFS-14 questionnaire variables are reported as mean and standard deviation. Qualitative data are expressed as frequency and percentage for patient’s gender, affected limb, cancer type, and treatment type, and HFS-14 grade distributions and compared statistically between both groups by Chi-square test. Paired t-test used to compare between pre- and post-treatment within group A and group B for VASs and HFS-14 questionnaire. Independent (unpaired) t-test used to compare between group A and group B at pre- and post-treatment for demographic data, VAS and HFS-14 questionnaire variables. All statistical analyses were significant at level of probability (P ≤ 0.05).
In the current study, a total of 40 patients with palmo plantar erythrodysesthesia syndrome associated with cancer therapy participated were participated and distributed randomly into two equal groups (20 patients/group). The results of patients’ demographic data (Table 1) showed that no statistical significant differences (P>0.05) in mean values of patients age (P=0.360), weight (P=0.120), height (P=0.819), and BMI (P=0.138), gender (P=1.000), affected limb (P=0.494), cancer type (P=0.491), and treatment type (P=0.580) between both groups (Table 1).
| Items | Groups | P-value | ||
|---|---|---|---|---|
| Group A (n=20) | Group B (n=20) | |||
| Quantitative variables | Mean ±SD | Mean ±SD | ||
| Age (year) | 36.50 ±10.94 | 39.30 ±7.94 | 0.360 | |
| Weight (kg) | 65.50 ±8.81 | 60.50 ±10.94 | 0.120 | |
| Height (cm) | 172.60 ±8.95 | 173.20 ±7.46 | 0.819 | |
| BMI (kg/m2) | 22.06 ±2.70 | 20.61 ±3.32 | 0.138 | |
| Qualitative variables | Number (percentage) | Number (percentage) | ||
| Gender | Females | 12 (60.00%) | 12 (60.00%) | 1.000 |
| Males | 8 (40.00%) | 8 (40.00%) | ||
| Affected limb | Right hand | 5 (25.00%) | 5 (25.00%) | 0.494 |
| Left hand | 3 (15.00%) | 7 (35.00%) | ||
| Right foot | 6 (30.00%) | 4 (20.00%) | ||
| Left foot | 6 (30.00%) | 4 (20.00%) | ||
| Cancer types | Brest cancer | 2 (10.00%) | 2 (10.00%) | 0.491 |
| Renal cancer | 4 (20.00%) | 2 (10.00%) | ||
| Pancreas cancer | 2 (10.00%) | 4 (20.00%) | ||
| Brain tumor | 4 (20.00%) | 6 (30.00%) | ||
| Leukemia | 4 (20.00%) | 4 (20.00%) | ||
| Acute leukemia | 4 (20.00%) | 2 (10.00%) | ||
| Treatment type | Chemotherapy | 14 (70.00%) | 14 (70.00%) | 0.580 |
| Chemo & radio therapy | 6 (30.00%) | 5 (25.00%) | ||
| Surgery then chemotherapy | 0 (0.00%) | 1 (5.00%) | ||
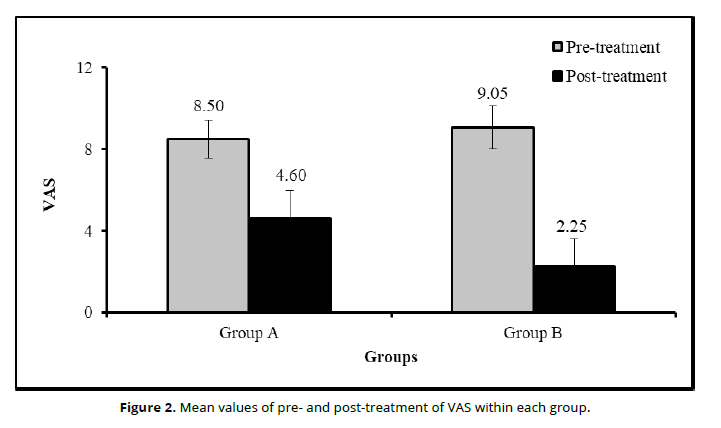
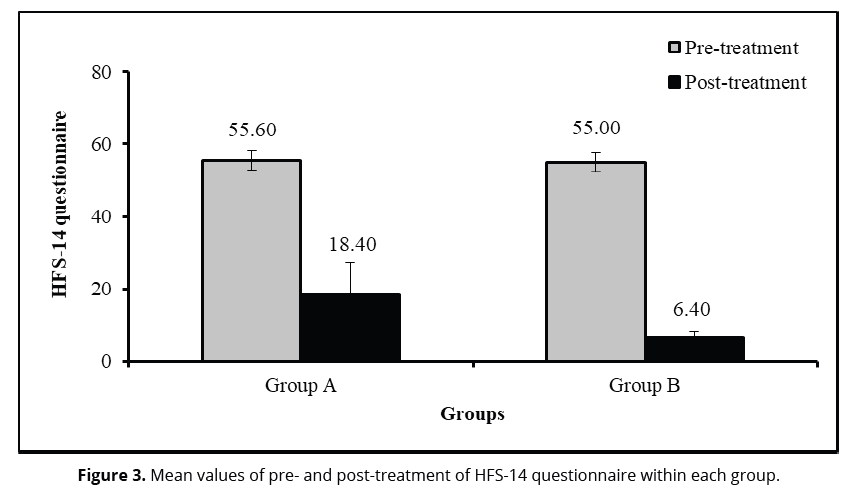
The statistical comparison for VAS (Table 2 and Figure 3) within each group revealed there were significantly (P<0.05) decreased in VAS at post-treatment compared to pre-treatment within group A (P=0.0001) and group B (P=0.0001). The change (MD) and improvement percentage of VAS due to time effect in group A were 3.90 and 45.88%, respectively; in group B were 6.80 and 75.13%, respectively. Moreover, HFS-14 questionnaire (Table 2 and Figure 3) significantly (P<0.05) decreased at post-treatment compared to pre-treatment within group A (P=0.0001) and Group B (P=0.0001). The change (MD) and improvement percentage of VAS due to time effect in group A were 37.20 and 66.90%, respectively; in group B were 48.60 and 88.36%, respectively.
| Variables | Items | Groups (Mean ±SD) | Change (MD) | P-value2 | |
|---|---|---|---|---|---|
| Group A (n=20) | Group B (n=20) | ||||
| VAS | Pre-treatment | 8.50 ±0.94 | 9.05 ±1.05 | 0.60 | 0.068 |
| Post-treatment | 4.60 ±1.39 | 2.25 ±1.33 | 2.35 | 0.0001* | |
| Change (MD) | 3.90 | 6.80 | |||
| Improvement % | 45.88% | 75.13% | 29.24 | 0.0001* | |
| P-value1 | 0.0001* | 0.0001* | |||
| HFS-14 questionnaire | Pre-treatment | 55.60 ±2.68 | 55.00 ±2.75 | 0.60 | 0.489 |
| Post-treatment | 18.40 ±8.74 | 6.40 ±2.02 | 12.00 | 0.0001* | |
| Change (MD) | 37.20 | 48.60 | |||
| Improvement % | 66.90% | 88.36% | 21.46 | 0.0001* | |
| P-value1 | 0.0001* | 0.0001* | |||
The statistical comparison for VAS and HFS-14 questionnaire between both groups revealed no statistical significant differences (P>0.05) in VAS (P=0.068; Table 2 and Figure 3) and HFS-14 questionnaire (P=0.489; Table 2 and Figure 3) at pre-treatment. However, at post-treatment, there were significant differences (P<0.05) in VAS (P=0.0001), improvement (in Group A = 45.88% vs Group B = 75.13%) of VAS (P=0.0001), and HFS-14 questionnaire (P=0.0001) as well as improvement ((in Group A = 66.90% vs Group B = 88.83) of HFS (p=0.0001) (Table 2).
Data are reported as mean ±standard deviation (SD); MD: Mean difference; CI: confidence interval; P-value: probability value; *Significant (P<0.05)
P-value1: Probability value within each group; P-value2: Probability value between both groups
(Figure 2, Figure 3)
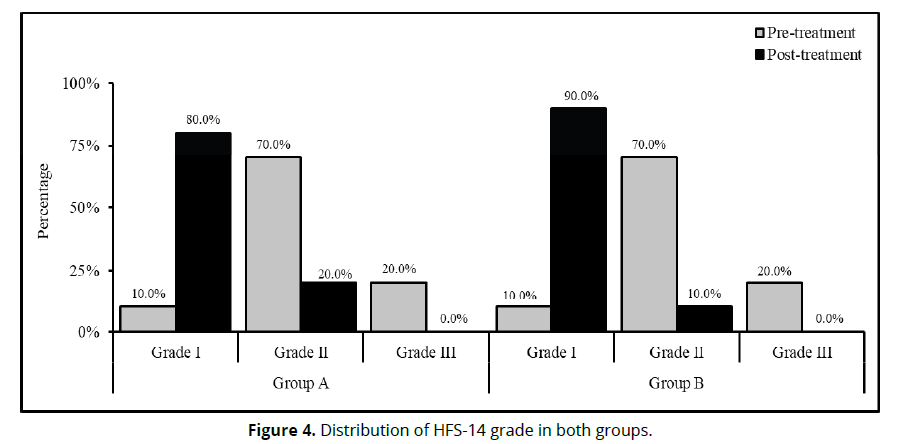
The statistical comparison for HFS-14 grade distribution (Table 3 and Figure 4) within each group revealed there were significant difference (P<0.05) in -14 grade distribution between pre- and post-treatment within group A (P=0.0001) and group B (P=0.0001). The statistical comparison for HFS-14 grade distribution (Table 3 and Figure 4) between both groups revealed no statistical significant differences (P>0.05) in HFS-14 grade distribution at pre-treatment (P=1.000) and post-treatment (P=0.376) (Table 3).
| Treatment | Items | Groups | P-value2 | |
|---|---|---|---|---|
| Group A (n=20) | Group B (n=20) | |||
| Pre-treatment | Grade I | 2 (10.00%) | 2 (10.00%) | 1.000 |
| Grade II | 14 (70.00%) | 14 (70.00%) | ||
| Grade III | 4 (20.00%) | 4 (20.00%) | ||
| Post-treatment | Grade I | 16 (80.00%) | 18 (90.00%) | 0.376 |
| Grade II | 4 (20.00%) | 2 (10.00%) | ||
| Grade III | 0 (0.00%) | 0 (0.00%) | ||
| P-value1 | 0.0001* | 0.0001* | ||
Data are reported as frequency (percentage) P-value: probability value;*Significant (P<0.05)
P-value1: Probability value within each group; P-value2: Probability value between both groups (Figure 4).
Discussion
This study aimed to evaluate the effectiveness of Photobiomodulation (PBM) therapy at two different fluence levels (2 J/cm² and 4 J/cm²) in managing Hand-Foot Syndrome (HFS) in cancer patients undergoing chemotherapy. The results showed significant improvements in pain reduction, symptom severity, functional abilities, and social participation for both treatment groups. However, Group B (4 J/cm²) consistently exhibited superior outcomes compared to Group A (2 J/cm²), highlighting a dose-dependent response. These findings align with previous studies, including those by Robijns et al 6 and Avci et al 7, who observed that higher fluencies of PBM are more effective in reducing inflammation and supporting tissue repair, both of which are critical in mitigating the painful manifestations of HFS.
The improved outcomes in Group B (4 J/cm²) are consistent with the findings of Zimmermann et al. 8, who noted enhanced analgesic and anti-inflammatory effects of PBM at moderate fluence levels. Similarly, Maiya et al 9 reported greater reductions in tissue injury scores when higher doses of PBM were administered. The current study further substantiates these findings by demonstrating that 4 J/cm² is more effective in improving functional abilities such as turning a key, preparing meals, and performing daily tasks, in line with Schindl et al. 10 and Bjordal et al. 11, who found that PBM enhances tissue repair and functional mobility in patients with chronic wounds or skin damage.
Pain reduction, a critical outcome of this study, was observed in both groups, with Group B showing a more significant improvement in pain scores. The Visual Analog Scale (VAS) pain scores in Group A dropped from 8.50 to 4.60, while in Group B, they decreased from 9.10 to 2.25, confirming the analgesic potential of PBM. These results support the conclusions of Zadik et al. 12, who found PBM effective in reducing chemotherapy-induced pain, and Hamblin 13, who emphasized that PBM modulates nociceptive pathways and inflammatory mediators to alleviate pain perception. Additionally, the work by Yousuf et al. 14 and Lopes-Martins et al. 15 supports the current study's findings that higher fluence levels contribute to greater pain relief through enhanced mitochondrial activity and increased ATP production.
The findings regarding sleep disturbances are also noteworthy, as both groups showed improvement, but Group B demonstrated a more pronounced reduction in sleep-related difficulties. Sleep quality, often impaired by pain and discomfort associated with HFS, improved significantly in Group B, with 75% of participants reporting no difficulty falling asleep post-treatment. These results are consistent with Mücke et al. 16, who showed that pain relief achieved through interventions like PBM is linked to improved sleep quality. Additionally, Silva et al. 17 and Leal Junior et al. 18 reported similar benefits, where higher fluence levels not only facilitated pain relief but also enhanced relaxation and recovery, which are crucial for improving sleep in chronic pain patients. Also, with a study by Othman et al. 19 who stated that PBM therapy is helpful in reducing collagen concentration, oxidation and histological abnormalities in a research trial regarding tendoachilles damage. The improvement in the oxidant/antioxidant balance may operate as a mediating factor that decrease fibrosis. In addition, Ahmed et al.20 reported photo bio modulation is an effective physical therapy modality for improving shoulder functional performance.
Social participation and work-related impairments were also significantly impacted by the PBM treatment, with both groups experiencing complete resolution of these issues. The total symptom sum score, and VAS pain scores were significantly lower in Group B, reflecting the broader impact of PBM therapy on the physical and psychosocial dimensions of HFS. This improvement in quality of life is consistent with findings by Dodd et al. 21 and Cleeland et al. 22 who demonstrated that effective symptom management leads to enhanced social and occupational functioning. Additionally, the reduction in work impairments and social difficulties observed in this study mirrors the conclusions of Pimenta et al. 23, who noted that symptom severity directly correlates with social engagement and quality of life in cancer patients.
Finally, the marked improvements in mobility-related tasks, including standing, walking, and seated posture, further underscore the efficacy of PBM in restoring functional mobility. Group B demonstrated a greater recovery in these areas, with nearly 90% reporting no difficulty in mobility tasks post-treatment. This aligns with the research of Huang et al. 24 and Clijsen et al. 25, who observed that PBM improves physical function by reducing inflammation and facilitating neuromuscular recovery in patients with musculoskeletal or soft tissue injuries. The dose-dependent nature of PBM’s effects on mobility is supported by Tomazoni and Leal-Junior 26, who found that moderate doses of PBM are particularly effective in improving joint mobility and gait speed in patients with chronic conditions.
In conclusion, this study confirms the clinical efficacy of PBM therapy, particularly at 4 J/cm², in alleviating Hand-Foot Syndrome (HFS) symptoms, reducing pain, improving functional abilities, enhancing sleep quality, and restoring social and work-related functions in cancer patients. The findings underscore the importance of dose optimization in PBM protocols for managing chemotherapy-induced dermatologic toxicity, supporting the integration of this non-invasive treatment into routine oncology care for improving patient quality of life.
Disclosure statement
No author has any financial interest or received any financial benefit from this research.
Conflict of interest
The authors state no conflict of interest.
References
Kwakman JJM, Elshot YS, Punt CJA, and Koopman M. (2020) Management of cytotoxic chemotherapy-induced hand-foot syndrome. Oncol Rev. 14(1):442.
Sibaud V, Dalenc F, Chevreau C, Roché H, Delord JP, Mourey L, et al. (2011) HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. The Oncologist. 16(10):1469–78.
Latifyan S, Genot MT, Fernez B, Scharll MF, and Klastersky JA. (2020) Use of low-level laser therapy (LLLT) or photobiomodulation (PBM) for the management of the hand-foot syndrome (HSF) or palmo-plantar erythrodysesthesia (PPED) associated with cancer therapy. Support Care Cancer. 28(7):3287–90.
Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, Bryja A, et al. (2020) Photobiomodulation—Underlying Mechanism and Clinical Applications. J Clin Med. 9(6):1724.
Venancio R de A, Camparis CM, and Lizarelli R de FZ. (2005) Low intensity laser therapy in the treatment of temporomandibular disorders: a double-blind study. J Oral Rehabil. 32(11):800–7.
Robijns J, Censabella S, Claes S, Hendrix A, Fierens K, Van Eycken L, et al. (2018) A prospective pilot study on the effect of low-level laser therapy in breast cancer patients suffering from radiation dermatitis. Support Care Cancer. 26(12):4265–72.
Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, et al. (2013) Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin Cutan Med Surg. 32(1):41–52.
Zimmermann CC, Marangoni RA, Ribeiro MS, and Ferreira LM. (2019) Effects of photobiomodulation in patients with breast cancer-related lymphedema: A randomized controlled trial. Photomed Laser Surg. 37(3):150–6.
Maiya AG, Kumar P, and Rao L. (2006) Effect of low level helium-neon (He-Ne) laser therapy in type 2 diabetic patients with infected ulcers: A comparative study. Photomed Laser Surg. 23(2):187–91.
Schindl A, Schindl M, Schindl L, Pernerstorfer-Schön H, and Schindl A. (2000) Low-intensity laser therapy: A review. Lasers Surg Med. 27(2):163–8.
Bjordal JM, Johnson MI, Iversen V, Aimbire F, and Lopes-Martins RÁB. (2006) Low-level laser therapy in acute pain: A systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg. 24(2):158–68.
Zaiem A, Hammamia SB, Aouinti I, Charfi O, Ladhari W, Kastalli S, et al. (2022) Hand-foot syndrome induced by chemotherapy drug: Case series study and literature review. Indian J Pharmacol. 54(3):208–15.
Hamblin MR. (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 4(3):337–61.
Yousuf A, Abul MS, Siddiqui MA, and Danish RK. (2022) The efficacy of low-level laser therapy in the management of musculoskeletal and neuropathic pain: A meta-analysis. Pain Manag Nurs. 23(2):157–65.
Lopes-Martins RÁB, Marcos RL, Leonardo PS, Prianti Jr ACG, Muscara MN, Aimbire F, et al. (2016) Effect of low-level laser therapy on the initial stages of tissue repair: A comparative study in rat Achilles tendon using different wavelengths. Lasers Surg Med. 48(5):500–8.
Mücke M, Cuhls H, Radbruch L, Baron R, Tölle T, Maier C, et al. (2016) A systematic review of the effectiveness of treatments for persistent pain in older people. J Pain Symptom Manage. 51(3):490–508.
Silva DF, De Souza NC, Soares BC, and Costa IMC. (2019) The influence of low-level laser therapy on quality of sleep in individuals with chronic pain. Sleep Sci. 12(3):175–81.
Leal Junior ECP, Lopes-Martins RÁB, Frigo L, De Marchi T, Rossi RP, and Bjordal JM. (2010) Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to postexercise recovery. J Orthop Sports Phys Ther. 40(8):524–32.
Eman M. Othman, Amr A. Elgrahi, Mohammad H. Ahmad, Rokaia A. Toson (2022): Scar prevention by low level laser therapy on surgical wound post hand flexor tendon repair. Fizjoterapia Polska; 22(5); 40-47. doi.org/10.56984/8ZG20A8mY
Ahmed AN, Othman EM, Soliman HE, Abouelnaga WA. (2021): Transcutaneous Electrical Nerve Stimulation versus Laser on Shoulder Functional Performance in Living Liver Donors. Egyptian Journal of Hospital Medicine. 7;85(1):3279-83.
Dodd MJ, Miaskowski C, and Paul SM. (2011) Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 38(6):E403–10.
Cleeland CS, Sloan JA, and Park JH. (2013) Symptom burden: A major contributor to poor quality of life in oncology. J Natl Cancer Inst Monogr. 2013(46):16–20.
Pimenta CA de M, Teixeira MJ, and Cruz Dalben JM. (2018) Cancer pain and quality of life: The role of symptom control. Braz J Nurs. 71(3):1048–53.
Huang YY, Chen ACH, Carroll JD, and Hamblin MR. (2009) Biphasic dose response in low level light therapy. Dose-Response. 7(4):358–83.
Clijsen R, Brunner A, Barbero M, Clarys P, and Taeymans J. (2017) Effects of low-level laser therapy on pain in patients with musculoskeletal disorders: A systematic review and meta-analysis. Eur J Phys Rehabil Med. 53(4):603–10.
Tomazoni SS, and Leal-Junior ECP. (2020) Effects of photobiomodulation therapy in functional outcomes of musculoskeletal disorders: An overview of systematic reviews. Lasers Med Sci. 35(3):559–66.
Tan S, Ong J, Choo J, et al. (2024) Prevalence and risk factors of hand-foot syndrome among colorectal cancer patients receiving capecitabine.Support Care Cancer.;32(5):243.
Sibaud V (2016). Dermatologic Reactions to Chemotherapy and Targeted Therapy in Cancer Patients: Hand–Foot Syndro me and Rash.Am J Clin Dermatol. 2016;17(5):425–436