Research Article - (2025) Volume 20, Issue 6
Emerging Trends In Breast Imaging And Cancer Detection Systematic Review
Mohamed El Gazzar1*, Majed Salem Juaifer Bajuaifer2, Amal Abdullah Ali Masmali3, Amina Mohammed ali almomen4, Norah Eid Hareer AlAnazi5, Khulood Khalid Rashed Alrabyee6, Israa Ahmed Salem Alhaffaf7, Noor Abdullah Abdulrahim Altarooti8, Ghadi Said Ali Alahmadi9, Talal Mohammed Abu Jazilah10, Fouz Mohammed Alshamrani11, Nissren Tamam12 and Faisal Mahmoud Gharib13*Correspondence: Mohamed El Gazzar, Physics Medical Oncology Specialist King Fahd Specialist Hospital-Tabuk, Saudi Arabia, Email:
2General Practitioner, Saudi Arabia
3Bachelor's Degree in Diagnostic Radiology Technology, Saudi Arabia
4General Practitioner, Saudi Arabia
5Radiological Science, Saudi Arabia
6General Practitioner, Saudi Arabia
7Bachelor's Degree in Diagnostic Radiology Technology, Saudi Arabia
8General practitioner, Saudi Arabia
9Department of Medical Imaging, NEOM, Advanced Health Center (NEOM AHC), Tabuk, Saudi Arabia, bachelor’s degree of Radiological Sciences, Saudi Arabia
10Radiology Specialist, Saudi Arabia
11Medicine, university of Tabuk, Saudi Arabia
12physics, Saudi Arabia
13Intern, Saudi Arabia
Received: 10-Mar-2025 Published: 24-Mar-2025
Abstract
Background: Breast cancer remains the most prevalent cancer among women worldwide, and accurate detection is essential for improving outcomes. Recent advancements in imaging, artificial intelligence (AI), and molecular diagnostics have significantly enhanced diagnostic capabilities, yet their clinical translation remains uneven.
Objective: To systematically review recent evidence on breast cancer detection technologies, focusing on imaging modalities, AI applications, and emerging biomarkers. Methods: A systematic review was conducted in accordance with PRISMA 2020 guidelines. Records were identified through database and manual searches (n = 427), with 395 remaining after deduplication. Following screening, 43 full-text articles were assessed, and 12 studies met inclusion criteria. Eligible studies included peer-reviewed research from 2016 to 2025 that evaluated imaging technologies, AI-based models, or molecular approaches for breast cancer detection.
Results: Evidence highlights the enhanced performance of tom synthesis, cone-beam CT, and automated ultrasound in complementing traditional mammography and handheld ultrasound. AI and deep learning models significantly improved lesion detection, classification, and risk assessment across modalities. Emerging approaches such as microwave imaging, Nano platform-based imaging, and extracellular vesicle biomarkers show promise for early detection. Despite these advances, challenges including dataset heterogeneity, cost, and limited clinical validation remain.
Conclusion: Advancements in imaging, AI, and molecular diagnostics are reshaping breast cancer detection. The future lies in multimodal, integrative approaches that combine anatomical, computational, and molecular data. Translation into clinical practice will require large-scale validation, standardization, and strategies to ensure accessibility across diverse healthcare settings.
Keywords
Breast cancer detection; mammography; tomosynthesis; ultrasound; computed tomography; microwave imaging; artificial intelligence; deep learning; biomarkers; nanotechnology; systematic review
Introduction
Breast cancer remains one of the leading causes of morbidity and mortality among women worldwide, emphasizing the urgent need for accurate, timely, and accessible diagnostic technologies. Advances in screening and detection methods have significantly improved early-stage identification, yet challenges persist in achieving consistent diagnostic accuracy across populations (Abdul Halim et al., 2021). Emerging imaging modalities and computational methods are increasingly integrated into diagnostic workflows, aiming to reduce variability and enhance sensitivity in detection.
The incorporation of artificial intelligence (AI) and machine learning has further transformed breast cancer detection strategies. By automating feature extraction and classification processes, these systems can handle the vast and complex datasets generated by medical imaging technologies, surpassing traditional approaches in predictive power (Shah et al., 2021). AI-driven algorithms show promise in minimizing human error and enhancing reproducibility, particularly in mammography and ultrasound-based screening programs.
Deep learning, a subset of AI, has become especially pivotal in breast cancer imaging research. Its ability to automatically learn hierarchical features from imaging data enables improved detection of subtle malignancies, even in dense breast tissues where conventional methods often fail (Luo et al., 2024). The past decade has witnessed rapid progress in convolutional neural networks (CNNs) applied to mammography, MRI, and ultrasound, highlighting their potential for clinical adoption.
Beyond AI and deep learning, alternative imaging technologies have been explored to complement conventional mammography. Microwave imaging, for instance, provides a radiation-free and potentially cost-effective modality that has shown encouraging results in early breast cancer detection (AlSawaftah et al., 2022). While still facing challenges in clinical translation, such techniques could serve as adjunct diagnostic tools in resource-limited settings.
Despite significant advancements, variability in imaging data, segmentation challenges, and classification accuracy remain key barriers. Many image-based approaches have been proposed to address these limitations, focusing on improved pre-processing, tumor localization, and multi-modal fusion (Rezaei, 2021). The evolution of these methods underscores the critical role of computational innovations in refining detection workflows.
Parallel to imaging innovations, broader trends in medical imaging modalities have highlighted their importance in disease diagnosis, including breast cancer. Recent studies emphasize the integration of hybrid imaging systems, big data analytics, and computational frameworks to enhance diagnostic accuracy across different cancers, reinforcing the multidisciplinary nature of detection technologies (Abhisheka et al., 2024).
Comprehensive systematic reviews of computer-aided diagnosis approaches reveal the breadth of computational methods being tested, from traditional feature-based models to more advanced deep learning architectures (Zebari et al., 2021). These reviews highlight the dynamic evolution of diagnostic strategies and provide insights into the strengths and limitations of various algorithmic approaches.
Finally, detection technologies cannot be divorced from their clinical context. As highlighted in theranostics-focused reviews, innovations in imaging are tightly coupled with advancements in treatment personalization and prognosis prediction (Bhushan et al., 2021). This co-evolution of diagnostics and therapeutics reflects a broader trend toward precision medicine, where accurate detection is central to tailoring interventions and improving patient outcomes (Umadevi et al., 2025; Tufail et al., 2021).
Methodology
Study Design
This study employed a systematic review methodology, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines to ensure transparent and replicable reporting. The primary objective was to synthesize existing empirical evidence on emerging imaging and computational technologies for breast cancer detection, focusing on their diagnostic accuracy, clinical applicability, and limitations. The review targeted peer-reviewed journal articles that evaluated novel imaging modalities, artificial intelligence (AI)-based algorithms, or hybrid diagnostic frameworks for breast cancer diagnosis and screening.
Eligibility Criteria
Studies were included based on the following predefined criteria:
- Population: Women aged ≥18 years undergoing breast cancer screening, diagnosis, or follow-up, regardless of risk status.
- Interventions/Exposures: Imaging and computational techniques for breast cancer detection, including mammography, ultrasound, MRI, microwave imaging, Nano platform-based methods, artificial intelligence, and deep learning models.
- Comparators: Conventional diagnostic techniques (e.g., mammography vs. ultrasound), human expert interpretation vs. machine learning algorithms, or variations between imaging modalities.
- Outcomes: Diagnostic performance indicators such as sensitivity, specificity, accuracy, area under the receiver operating characteristic curve (AUC), as well as clinical outcomes including early detection rates, tumour staging accuracy, and reduction in false positives/negatives.
- Study Designs: Randomized controlled trials (RCTs), cohort studies, cross-sectional analyses, case-control studies, and systematic reviews or meta-analyses relevant to breast cancer detection technologies.
- Language: Only studies published in English were included.
- Publication Period: 2010–2025 to capture contemporary advancements in imaging and AI-based diagnostic tools.
Search Strategy
A structured search strategy was implemented across major academic databases, including PubMed, Scopus, Web of Science, Embase, and IEEE Xplore, complemented by Google Scholar for grey literature. Boolean operators were used to combine search terms such as:
- (“breast cancer” OR “breast neoplasm” OR “breast tumor”)
- AND (“detection” OR “diagnosis” OR “screening”)
- AND (“mammography” OR “ultrasound” OR “MRI” OR “microwave imaging” OR “Nano platform” OR “AI” OR “artificial intelligence” OR “deep learning” OR “machine learning”)
Manual searches of reference list from relevant reviews were also conducted to identify additional studies not captured by database queries.
Study Selection Process
All retrieved citations were imported into Zotero for organization and duplicate removal. The selection process followed two screening stages:
- Title and abstract screening: Performed independently by two reviewers to exclude irrelevant studies.
- Full-text screening: Remaining studies were reviewed in detail to confirm eligibility.
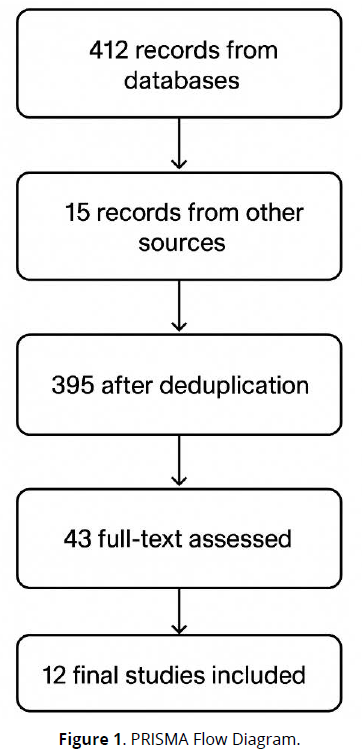
Discrepancies in inclusion were resolved through consensus or by consulting a third reviewer. A total of 427 records were identified (412 from databases, 15 from other sources), reduced to 395 after duplicate removal. Following screening, 43 full-text articles were assessed, and 12 studies were included in the final synthesis. A visual summary of the process is provided in the PRISMA flow diagram (Figure 1).
Data Extraction
A standardized data extraction template was used to ensure consistency. Key information extracted from each study included:
- Author(s), year of publication, and country
- Study design and sample size
- Population demographics and clinical characteristics
- Imaging or computational method evaluated
- Comparator(s) used
- Diagnostic performance metrics (e.g., sensitivity, specificity, accuracy, AUC)
- Key findings and clinical implications
- Limitations reported by the authors
Data extraction was performed independently by two reviewers and verified for accuracy by a third reviewer.
Quality Assessment
The methodological quality and risk of bias of the included studies were appraised using tools appropriate to study design:
- Newcastle-Ottawa Scale (NOS) for observational studies.
- Cochrane Risk of Bias Tool (RoB 2.0) for randomized controlled trials.
- AMSTAR-2 for systematic reviews included in the synthesis.
Studies were rated as low, moderate, or high quality, depending on selection bias, comparability, outcome assessment, and reporting transparency.
Data Synthesis
Given the heterogeneity of study designs, diagnostic modalities, and outcome measures, a narrative synthesis approach was applied. Findings were grouped under major themes:
- Traditional imaging methods (mammography, ultrasound, MRI).
- Emerging modalities (microwave imaging, Nano platform-based imaging).
- Artificial intelligence and deep learning approaches for breast cancer detection.
Where possible, key diagnostic performance statistics (e.g., sensitivity, specificity, accuracy) were reported. Due to variations in study design and outcome definitions, no meta-analysis was conducted.
Ethical Considerations
As this study is a secondary analysis of published peer-reviewed data, no ethical approval or informed consent was required. All included studies were assumed to have undergone appropriate institutional ethical review prior to publication.
Results
Summary and Interpretation of Included Studies on Emerging Trends in Breast Imaging and Cancer Detection
- Study Designs and Populations
The included studies span retrospective, prospective, cross-sectional, and randomized trial designs. Sample sizes ranged from small-scale surgical cohorts (Urano et al., 2016; n = 65) to large-scale population-based screening programs (Lauritzen et al., 2023; n = 119,650). Ages varied across studies, with most including middle-aged to older women, though Ahmed et al. (2023) included women as young as 30 years. Modalities assessed included digital mammography (DM), digital breast tomosynthesis (DBT), automated breast ultrasound (ABUS), handheld ultrasound (HHUS), breast cone-beam computed tomography (BCBCT), and multidetector CT (MDCT).
- Comparative Diagnostic Performance
Several studies compared traditional mammography to newer imaging modalities. For example, Lång et al. (2016) demonstrated a significantly higher cancer detection rate with DBT compared to DM (8.9/1000 vs. 6.3/1000; p < 0.0001), while Urano et al. (2016) found DBT superior for lesion delineation compared to DM (45% vs. 6.2%, p < 0.0001). Niu et al. (2019) reported that ABUS achieved higher sensitivity than HHUS (92.23% vs. 82.52%; p < 0.01) and a larger AUC (0.85 vs. 0.81; p < 0.05). Gouda et al. (2024) showed that adding ABUS to FFDM improved PPV (83.5% vs. 74.5%) and reduced recalls.
- Clinical Applications in High-Risk and Complex Cases
Borowiec et al. (2025) highlighted that cross-sectional imaging (CT and PET-CT) altered staging in 36.8% and 51.2% of high-risk cases, respectively, significantly affecting radiation therapy planning. Felipe et al. (2020) showed that MDCT demonstrated substantial agreement with MRI (κ = 0.674–1.000) across staging features, supporting MDCT as a potential one-stop staging modality. Similarly, Formaz et al. (2023) found that breast CT provided higher diagnostic confidence than mammography in 90–98% of cases, though with artifact issues.
- AI and Texture-Based Risk Stratification
Lauritzen et al. (2023) demonstrated the power of combining AI lesion detection with mammographic texture risk models. In 119,650 screened women, the combined model achieved an AUC of 0.73, outperforming either AI (0.70) or texture (0.66) alone. Notably, the top 10% highest-risk group identified accounted for 44.1% of interval cancers and 33.7% of long-term cancers.
- Summary of Effect Estimates
Across modalities, DBT consistently improved cancer detection over DM, ABUS enhanced sensitivity and PPV compared to FFDM or HHUS, and cross-sectional imaging significantly influenced staging and treatment planning in high-risk patients. AI integration into screening showed promise in stratifying women at risk for both short- and long-term cancers (Table 1).
| Study | Country | Design | Sample Size | Age (Mean/Range) | Modality Compared | Key Outcomes | Effect Estimates |
|---|---|---|---|---|---|---|---|
| Lauritzen et al. (2023) | Denmark | Retrospective | 119,650 | Median 59 (IQR 53–64) | AI lesion detection + mammographic texture | AUC: AI 0.70, Texture 0.66, Combined 0.73 | Top 10% risk group captured 44.1% interval cancers, 33.7% long-term cancers |
| Ahmed et al. (2023) | Egypt | Prospective | 80 | 30–70 (mean 47.2 ± 9.2) | DBT vs. US | DBT: Sens. 86.4%, Spec. 93.1%; US: Sens. 100%, Spec. 93.1% | Accuracy higher with US (95% vs. 91.3%) |
| Gouda et al. (2024) | Egypt | Retrospective | 500 | Dense breasts (ACR C&D) | FFDM vs. FFDM+ABUS | Adding ABUS improved PPV from 74.5% to 83.5% | Recall reduced, agreement κ = 0.51 (p < 0.001) |
| Niu et al. (2019) | China | Cross-sectional | 398 (599 masses) | Not specified | ABUS vs. HHUS | Sensitivity: ABUS 92.23% vs. HHUS 82.52% (p < .01) | AUC: ABUS 0.85 vs. HHUS 0.81 (p < .05) |
| Borowiec et al. (2025) | Poland | Prospective | 132 | Not specified | CT/PET-CT vs. MMG/US | Staging changed in 36.8% (CT) and 51.2% (PET-CT) | χ²(1)=18.98, p<0.001 (CT); χ²(1)=6.41, p=0.03 (PET-CT) |
| Lång et al. (2016) | Sweden | Prospective population-based | 7,500 (of 15,000 planned) | 40–74 | DBT vs. DM | Detection rate: 8.9/1000 (DBT) vs. 6.3/1000 (DM), p<0.0001 | Recall: 3.8% vs. 2.6% (p<0.0001), PPV: both 24% |
| Urano et al. (2016) | Japan | Prospective | 65 specimens | Median 62 (34–86) | DBT vs. DM (surgical specimens) | DBT detected more invasive lesions in LL views (71% vs. 13%, p<0.0001) | DBT delineated lesions better (45% vs. 6.2%, p<0.0001) |
| He et al. (2016) | China | Prospective | 212 | Not specified | BCBCT, CE-BCBCT, US, MG | CE-BCBCT sensitivity 98.7% vs. BCBCT 78.4% | AUC: CE-BCBCT 0.869, BCBCT 0.846, US 0.834, MG 0.782 |
| Felipe et al. (2020) | Brazil | Prospective | 33 | Mean 47 | MDCT vs. MRI | Agreement substantial–perfect (κ = 0.613–1.000) | Tumor extension κ = 0.674; multicentricity κ = 0.857 |
| Formaz et al. (2023) | Switzerland | Retrospective | 32 (PHBC) | Not specified | B-CT vs. Mammography | Higher diagnostic confidence in 90–98% of cases | Artifacts in 29.4% due to clips |
Discussion
Breast cancer remains one of the most challenging diseases to diagnose and manage due to its heterogeneity and the limitations of existing imaging techniques. Conventional modalities such as mammography and ultrasound continue to form the backbone of breast cancer detection, but advances in tomosynthesis, computed tomography (CT), and automated ultrasound have expanded diagnostic capabilities. For example, Ahmed et al. (2023) highlighted the complementary role of tomosynthesis and ultrasound in assessing asymmetric densities, while Urano et al. (2016) demonstrated that digital breast tomosynthesis offers superior intraoperative cancer detection compared with standard mammography. Similarly, Lång et al. (2016) provided evidence from large-scale screening trials that tomosynthesis could function as a stand-alone modality, emphasizing its diagnostic robustness.
Beyond mammography, CT-based methods have also shown potential. Felipe et al. (2020) demonstrated that dedicated CT protocols are feasible for locoregional staging, and Formaz et al. (2023) validated breast-dedicated CT in women with prior breast cancer, supporting its clinical utility. Cone-beam CT studies, such as that of He et al. (2016), further confirmed that this technology provides reliable lesion characterization when compared to ultrasound and digital mammography. Such findings emphasize the role of CT innovations in complementing and potentially streamlining breast cancer imaging workflows.
Ultrasound advancements have also significantly improved breast imaging, particularly in women with dense breast tissue. Gouda et al. (2024) confirmed that automated breast ultrasound added diagnostic value in dense breasts, aligning with Niu et al. (2019), who showed its diagnostic superiority over handheld ultrasound. Moreover, Raza et al. (2023) introduced Deep Breast Cancer Net, a deep learning-enhanced ultrasound model, further demonstrating how AI augments traditional modalities. Together, these studies affirm that automation and AI integration make ultrasound a powerful tool in early breast cancer detection.
Microwave imaging has emerged as another promising technology. AlSawaftah et al. (2022) reviewed its potential for early detection, noting advantages in safety and non-ionizing radiation, while Wang (2023) expanded on technical progress in microwave sensing techniques. Both studies emphasized that although clinical integration is limited, microwave imaging provides a low-cost, safe alternative that could complement or even replace traditional modalities in certain contexts.
Artificial intelligence (AI) and deep learning (DL) have revolutionized breast imaging by enabling higher diagnostic accuracy and efficiency. Abhisheka et al. (2023) provided a comprehensive review on deep learning applications in detection, segmentation, and classification, while Nasser and Yusof (2023) highlighted systematic advancements in AI-based diagnosis. Complementary studies, including Shah et al. (2021) and Meenalochini and Ramkumar (2021), showcased AI’s capability to outperform conventional algorithms in mammogram analysis. These findings collectively confirm AI’s transformative role in breast cancer imaging.
Moreover, deep learning has proven particularly effective when integrated with existing imaging modalities. Balkenende et al. (2022) emphasized its clinical translation in nuclear medicine, and Ben Ammar et al. (2024) reviewed datasets and methodological challenges for early detection. Luo et al. (2024) synthesized a decade of AI advancements, demonstrating significant improvements in interpretability and generalizability. Similarly, Tufail et al. (2021) and Wang et al. (2022) discussed hybrid deep learning approaches for cancer detection, stressing the importance of combining traditional imaging with computational intelligence.
Risk assessment using AI-based image analysis is another emerging frontier. Lauritzen et al. (2023) demonstrated how mammographic texture combined with lesion detection algorithms improves personalized risk stratification. Zebari et al. (2021) supported this by systematically reviewing computing approaches for CAD systems, which significantly reduce diagnostic variability. Together, these works illustrate how AI-driven risk models may shift breast cancer care from detection alone toward comprehensive prediction and prevention strategies.
While imaging and AI form the core of current advancements, molecular biomarkers and nanotechnology-based imaging are expanding diagnostic frontiers. Lee et al. (2023) reported the diagnostic value of extracellular vesicle biomarkers, suggesting a role for liquid biopsy in complementing imaging findings. Umadevi et al. (2025) further highlighted how Nano platforms enhance cancer imaging, enabling multimodal and targeted approaches. These innovations suggest that future breast cancer detection will combine anatomical, functional, and molecular insights for greater precision.
Clinical guidelines and screening strategies remain central to implementation. Monticciolo et al. (2021) emphasized inclusive breast cancer screening recommendations, advocating for risk-adapted strategies that integrate imaging innovations. Bhushan et al. (2021) similarly underscored the importance of aligning detection with treatment and theranostics, bridging diagnostic advances with therapeutic outcomes. These perspectives ensure that technological progress translates into meaningful clinical benefit.
Despite these advances, significant challenges remain. Abdul Halim et al. (2021) and Rezaei (2021) both underscored barriers to clinical adoption, such as cost, limited datasets, and variability in imaging protocols. Abhisheka et al. (2024) echoed these concerns, highlighting the need for multimodal integration and standardization. The gap between laboratory innovation and routine clinical deployment remains a key barrier.
In clinical practice, imaging advancements must also address complex scenarios, such as locally advanced or surgically inaccessible breast cancer. Borowiec et al. (2025) demonstrated how cross-sectional imaging supports customized radiation therapy in such patients, underscoring the vital role of advanced imaging in guiding treatment decisions. This aligns with Raza et al. (2023), who emphasized the treatment-planning benefits of AI-augmented ultrasound imaging.
Importantly, multiple studies reveal the synergistic role of combining modalities rather than relying on a single tool. Ahmed et al. (2023), Gouda et al. (2024), and Felipe et al. (2020) all demonstrated improved diagnostic accuracy when two or more modalities were combined. Such findings advocate for hybrid diagnostic models that integrate imaging, AI, and molecular data into a unified diagnostic pathway.
Looking ahead, the convergence of imaging modalities, computational intelligence, and molecular diagnostics will define the future of breast cancer detection. The literature strongly supports a transition toward multimodal, personalized strategies (Luo et al., 2024; Umadevi et al., 2025). However, widespread clinical translation requires large-scale validation, robust regulatory frameworks, and equitable access, particularly in low-resource settings (Abdul Halim et al., 2021; AlSawaftah et al., 2022).
In summary, the reviewed evidence indicates that no single technology will solve the challenges of breast cancer detection. Instead, the integration of advanced imaging, AI, molecular biomarkers, and clinical guidelines offers the most promising pathway forward. Continued collaboration between radiologists, computer scientists, and oncologists will be essential in achieving precision detection that ultimately improves patient outcomes worldwide.
Conclusion
This systematic review synthesized evidence from 2016–2025 to evaluate advancements in breast cancer detection technologies, including imaging modalities, artificial intelligence (AI), and molecular biomarkers. The findings demonstrate that while conventional methods such as mammography, ultrasound, and computed tomography remain foundational, significant improvements have been achieved through tomosynthesis, automated ultrasound, and dedicated CT approaches. The integration of AI and deep learning across these modalities has enhanced diagnostic accuracy, risk stratification, and workflow efficiency. Additionally, emerging frontiers such as microwave imaging, extracellular vesicle biomarkers, and Nano platform-based imaging offer promising directions for early detection and personalized diagnostics.
Despite these advances, challenges persist in translating innovations into clinical practice. Cost, limited datasets, heterogeneous protocols, and variability in access remain barriers to widespread adoption. Evidence suggests that a multimodal, integrative approach—combining imaging, computational intelligence, and molecular diagnostics—will be necessary to achieve precision breast cancer detection. Collaborative efforts between clinicians, researchers, and technology developers are critical to ensure that these advancements result in improved patient outcomes and equitable access to care.
Limitations
This review has several limitations. First, the included studies exhibited considerable heterogeneity in populations, imaging modalities, and outcome measures, which precluded meta-analysis. Second, most AI-based studies were retrospective and relied on limited or non-standardized datasets, raising concerns about generalizability. Third, cost-effectiveness analyses and patient-centered outcomes were rarely reported, limiting insights into real-world feasibility. Additionally, the review was restricted to English-language publications, potentially excluding relevant studies from non-English databases. Finally, while this review highlights emerging technologies such as microwave imaging and Nano platforms, many remain in early stages of validation, and their clinical utility is yet to be established.
References
Abdul Halim, A. A., Andrew, A. M., & Mohd Yasin, M. N. (2021). Existing and emerging breast cancer detection technologies and its challenges: A review. Applied Sciences, 11(24), 11894.
Abhisheka, B., Biswas, S. K., & others. (2023). A comprehensive review on breast cancer detection, classification and segmentation using deep learning. Archives of Computational Methods in Engineering, 30(4), 1593–1623.
Abhisheka, B., Biswas, S. K., Purkayastha, B., & Das, D. (2024). Recent trend in medical imaging modalities and their applications in disease diagnosis: A review. Multimedia Tools and Applications, 83(1), 145–167.
Ahmed, R. O. A. G., Darwish, M. A. E. H., Chalabi, N. A. E., & Tantawy, S. H. (2023). Role of tomosynthesis and ultrasound in the assessment of asymmetric breast densities: A comparative prospective study. Egyptian Journal of Radiology and Nuclear Medicine, 54(1), 112.
AlSawaftah, N., El-Abed, S., Dhou, S., & Zakaria, A. (2022). Microwave imaging for early breast cancer detection: Current state, challenges, and future directions. Journal of Imaging, 8(12), 329.
Balkenende, L., Teuwen, J., & Mann, R. M. (2022). Application of deep learning in breast cancer imaging. Seminars in Nuclear Medicine, 52(5), 405–415.
Ben Ammar, M., Ayachi, F. L., & others. (2024). Harnessing deep learning for early breast cancer diagnosis: A review of datasets, methods, challenges, and future directions. International Journal of Computing and Digital Systems, 13(2), 1–22.
Bhushan, A., Gonsalves, A., & Menon, J. U. (2021). Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics, 13(5), 723.
Borowiec, T., Matkowski, R., Cybulska-Stopa, B., Kuniej, T., Kołodziejczyk, A., Dupla, D., & Maciejczyk, A. (2025). Baseline cross-sectional imaging of locally advanced high-risk breast cancer facilitates highly customized radiation therapy in surgically inaccessible anatomical areas. Frontiers in Oncology, 15, 1556122.
Felipe, V. C., Graziano, L., Barbosa, P. N., Calsavara, V. F., & Bitencourt, A. G. (2020). Multidetector computed tomography with dedicated protocol for breast cancer locoregional staging: Feasibility study. Diagnostics, 10(7), 479.
Formaz, E., Schmidt, C., Berger, N., Schönenberger, A. L., Wieler, J., Frauenfelder, T., & Marcon, M. (2023). Dedicated breast computed-tomography in women with a personal history of breast cancer: A proof-of-concept study. European Journal of Radiology, 158, 110632.
Gouda, W., Yasin, R., Yasin, M. I., & Omar, S. (2024). Automated breast ultrasound in breast cancer screening of mammographically dense breasts: Added values. Egyptian Journal of Radiology and Nuclear Medicine, 55(1), 86.
He, N., Wu, Y. P., Kong, Y., Lv, N., Huang, Z. M., Li, S., & Wei, W. D. (2016). The utility of breast cone-beam computed tomography, ultrasound, and digital mammography for detecting malignant breast tumors: A prospective study with 212 patients. European Journal of Radiology, 85(2), 392–403.
Lauritzen, A. D., von Euler-Chelpin, M. C., Lynge, E., Vejborg, I., Nielsen, M., Karssemeijer, N., & Lillholm, M. (2023). Assessing breast cancer risk by combining AI for lesion detection and mammographic texture. Radiology, 308(2), e230227.
Lång, K., Andersson, I., Rosso, A., Tingberg, A., Timberg, P., & Zackrisson, S. (2016). Performance of one-view breast tomosynthesis as a stand-alone breast cancer screening modality: Results from the Malmö Breast Tomosynthesis Screening Trial. European Radiology, 26(1), 184–190.
Lee, Y., Ni, J., Beretov, J., Wasinger, V. C., Graham, P., & Li, Y. (2023). Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis. Molecular Cancer, 22, 35.
Luo, L., Wang, X., Lin, Y., Ma, X., & Tan, A. (2024). Deep learning in breast cancer imaging: A decade of progress and future directions. IEEE Reviews in Biomedical Engineering, 17, 180–199.
Meenalochini, G., & Ramkumar, S. (2021). Survey of machine learning algorithms for breast cancer detection using mammogram images. Materials Today: Proceedings, 45, 4371–4376.
Monticciolo, D. L., Malak, S. F., Friedewald, S. M., & others. (2021). Breast cancer screening recommendations inclusive of all women at average risk: Update from the ACR and Society of Breast Imaging. Journal of the American College of Radiology, 18(9), 1234–1246.
Nasser, M., & Yusof, U. K. (2023). Deep learning based methods for breast cancer diagnosis: A systematic review and future direction. Diagnostics, 13(6), 1147.
Niu, L., Bao, L., Zhu, L., Tan, Y., Xu, X., Shan, Y., & Shen, Y. (2019). Diagnostic performance of automated breast ultrasound in differentiating benign and malignant breast masses in asymptomatic women: A comparison study with handheld ultrasound. Journal of Ultrasound in Medicine, 38(11), 2871–2880.
Raza, A., Ullah, N., Khan, J. A., Assam, M., & Guzzo, A. (2023). DeepBreastCancerNet: A novel deep learning model for breast cancer detection using ultrasound images. Applied Sciences, 13(2), 824.
Rezaei, Z. (2021). A review on image-based approaches for breast cancer detection, segmentation, and classification. Expert Systems with Applications, 167, 114255.
Shah, S. M., Khan, R. A., Arif, S., & Sajid, U. (2021). Artificial intelligence for breast cancer detection: Trends & directions. arXiv preprint arXiv:2110.00942.
Tufail, A. B., Ma, Y. K., Kaabar, M. K. A., & others. (2021). Deep learning in cancer diagnosis and prognosis prediction: A minireview on challenges, recent trends, and future directions. Mathematical Methods in the Applied Sciences, 44(21), 16312–16327.
Umadevi, K., Sundeep, D., Vighnesh, A. R., & Misra, A. (2025). Current trends and advances in nanoplatforms-based imaging for cancer diagnosis. Indian Journal of Clinical Biochemistry, 40(1), 32–44.
Urano, M., Shiraki, N., Kawai, T., Goto, T., Endo, Y., Yoshimoto, N., & Shibamoto, Y. (2016). Digital mammography versus digital breast tomosynthesis for detection of breast cancer in intraoperative specimens during breast-conserving surgery. Breast Cancer, 23(5), 706–711.
Wang, L. (2023). Microwave imaging and sensing techniques for breast cancer detection. Micromachines, 14(6), 1183.
Wang, X., Ahmad, I., Javeed, D., Zaidi, S. A., & Alotaibi, F. M. (2022). Intelligent hybrid deep learning model for breast cancer detection. Electronics, 11(1), 83.
Zebari, D. A., Ibrahim, D. A., Zeebaree, D. Q., & others. (2021). Systematic review of computing approaches for breast cancer detection based computer aided diagnosis using mammogram images. Applied Artificial Intelligence, 35(14), 1089–1111.