Research Article - (2025) Volume 20, Issue 5
*Correspondence: Moamen Abdelfadil Ismail, Consultant, King Abdulaziz Specialist Hospital-Sakaka- Aljouf, Saudi Arabia, Email:
2Otorhinolaryngology (ENT), Kuwait
3General practitioner, Saudi Arabia
4General Practitioner, Saudi Arabia
5Medical Student, College of Medicine, King Khalid University, Abha, Saudi Arabia
6College of medicine, Ibn Sina National College, Jeddah, Saudi Arabia
7General Practitioner, Aseer Health Cluster, Saudi Arabia
8Department of otolaryngology head and neck surgery, Armed Forces Hospital Southren Region, Khamis Mushait, Saudi Arabia
9Audioloist, Saudi Arabia
10Ent Resident, Saudi Arabia
11Medicine, Saudi Arabia
12Mbchb Dol Ent, Saudi Arabia
13Medical doctor, Saudi Arabia
14GP at KAUH MAKKAH, Saudi Arabia
Received: 01-Jul-2025 Published: 17-Aug-2025
Abstract
Background: Hearing loss is highly prevalent among older adults and is associated with cognitive decline, social isolation, and reduced quality of life. Cochlear implantation (CI) is increasingly utilized as an intervention for severe-to-profound hearing loss in this population. This review systematically examines outcomes related to auditory function, cognitive performance, and quality of life after CI in older adults.
Objectives: To synthesize and critically evaluate empirical evidence regarding the efficacy, cognitive benefits, safety, and psychosocial outcomes of cochlear implantation in individuals aged 60 years and older.
Methods: A systematic review methodology was employed, following PRISMA 2020 guidelines. Searches were conducted across PubMed, Scopus, Web of Science, and Embase for peer-reviewed studies published between 2010 and 2024. Inclusion criteria encompassed studies with older adult populations undergoing CI, reporting on at least one of the following: cognitive outcomes, speech perception, QoL metrics, or safety. Both qualitative and quantitative studies were included.
Results: Twenty-two studies met inclusion criteria. CI in older adults resulted in consistent improvements in speech recognition, verbal communication, emotional well-being, and cognitive function. These benefits were observed across varied age ranges, including the very elderly. Comorbidity presence did not substantially increase surgical risk. Several studies reported enhanced executive function, memory, and decreased depressive symptoms following implantation.
Conclusions: Cochlear implantation offers substantial benefits for elderly patients, both in auditory and non-auditory domains. Age alone should not preclude candidacy. Comprehensive geriatric assessments and standardized referral pathways may enhance access and outcomes. Future research should emphasize long term follow-up, cognitive subdomains, and equity in care delivery.
Keywords
Cochlear implantation; Older adults; Cognitive decline; Quality of life; Speech perception; Age-related hearing loss; Neuroplasticity; Audiological rehabilitation; Systematic review; Geriatric otology
Introduction
The global population is aging rapidly, and the prevalence of age-related hearing loss is rising in parallel. Presbycusis, or age-related sensorineural hearing loss, is among the most prevalent chronic health issues affecting older adults and has been linked to reduced communication ability, social isolation, and cognitive decline (Borre et al., 2023). For individuals with severe-to-profound bilateral hearing loss, conventional hearing aids often offer limited benefit due to insufficient amplification or poor speech discrimination. As a result, cochlear implantation (CI) is increasingly recognized as a clinically effective intervention that warrants critical evaluation in geriatric populations.
Historically, cochlear implantation in older adults was approached with caution. Concerns about surgical risks, age-related neuroplasticity limitations, comorbidities, and difficulties in device acclimatization contributed to underutilization (Sampathkumar et al., 2021). However, recent advances in surgical methods, miniaturized electrode arrays, and individualized mapping strategies have substantially improved the safety and efficacy of CI in the elderly. Expanding candidacy guidelines now routinely include patients in their eighties and nineties who previously would not have been considered viable candidates (Kay-Rivest et al., 2022).
Current literature increasingly supports the notion that cochlear implantation can lead to marked improvements in auditory outcomes, functional communication, and psychosocial well-being in older adults (Hamerschmidt et al., 2023). While early evidence was limited by outdated single-channel devices and narrow inclusion criteria, contemporary studies utilize robust speech-in-noise testing, real-world auditory assessments, and cognitive evaluations. Longitudinal designs further enable the tracking of sustained benefits and device usage beyond initial rehabilitation (An et al., 2023).
One of the most compelling motivations for cochlear implantation in older adults is its potential to slow or reverse cognitive decline. Untreated hearing loss has been identified as one of the most significant modifiable risk factors for dementia and cognitive deterioration (Calvino et al., 2022). By restoring auditory input, cochlear implants may reduce cognitive load, improve environmental awareness, and promote social engagement—factors closely linked with cognitive preservation. Meta-analyses demonstrate that CI users can achieve statistically significant improvements in executive function, working memory, and verbal recall even within the first year post-implantation (An et al., 2023; Hamerschmidt et al., 2023).
Quality of life (QoL) is another domain where elderly CI recipients show consistent improvement. Studies report enhanced communication ability, greater social interaction, improved emotional stability, and a stronger sense of independence following implantation (Andries et al., 2021). Disease-specific measures such as the Nijmegen Cochlear Implant Questionnaire (NCIQ) and the Hearing Handicap Inventory for the Elderly (HHIE) often show more consistent improvements than general QoL instruments like the SF-36. However, mental health and sensory functioning domains within generic tools do exhibit positive change in many cases (Cuda et al., 2024).
Despite these encouraging outcomes, several barriers to cochlear implantation in the elderly remain. Many older adults are not referred for CI evaluation due to misconceptions about age-based candidacy, surgical safety concerns, or a lack of awareness among healthcare providers (Kay-Rivest et al., 2022). Socioeconomic disparities, logistical challenges, and variable insurance coverage further limit access for many elderly individuals, particularly those from rural or underserved populations (Borre et al., 2023).
Another significant consideration is the role of comorbidities. Older adults often present with chronic conditions such as cardiovascular disease, diabetes, and mild cognitive impairment that may complicate candidacy assessments or postoperative outcomes (Sampathkumar et al., 2021). Nonetheless, evidence suggests that with appropriate perioperative management, complication rates in elderly CI recipients are not significantly different from those in younger populations. A review by Zia et al. (2021) found that surgical risks, including infections or device failure, remain low, and most patients recover without adverse long-term effects.
Given the growing demand for effective hearing rehabilitation in aging societies, there is a clear need to synthesize the latest evidence regarding cochlear implantation outcomes in elderly adults. This systematic review aims to assess the effectiveness, safety, cognitive benefits, and quality of life outcomes associated with cochlear implantation in adults aged 60 years and older, using findings from recent high-quality peer-reviewed studies.
Methodology
Study Design
This study employed a systematic review methodology consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines to ensure transparent, comprehensive, and replicable reporting. The primary objective of this review was to synthesize high-quality empirical evidence on the outcomes of cochlear implantation (CI) in elderly adults aged 60 years and older diagnosed with severe-to-profound bilateral sensorineural hearing loss. Specific outcomes assessed included speech recognition improvement, cognitive function, quality of life (QoL), and surgical safety profiles post-implantation. Only peer-reviewed articles involving human subjects and reporting clinical or psychological endpoints post-CI were included in the final synthesis.
Eligibility Criteria
Studies were considered eligible based on the following inclusion and exclusion criteria:
- Population: Adults aged 60 years and older diagnosed with bilateral severe-to-profound sensorineural hearing loss who received unilateral or bilateral cochlear implants.
- Interventions: Cochlear implantation using multichannel devices, irrespective of brand or surgical technique.
- Comparators: Either pre-implant scores of the same participants (longitudinal designs), age-matched non-implanted controls, or other patient subgroups (e.g., younger adults or different etiologies).
Outcomes
- Speech perception outcomes (e.g., sentence recognition, monosyllabic word tests, AzBio, CAP scores)
- Cognitive function changes (e.g., executive function, memory, attention)
- Quality of life assessments (e.g., NCIQ, HHIE, HUI3, WHOQOL-OLD)
- Psychological status (e.g., depression, anxiety, loneliness)
- Device retention or continued use
- Complications (minor and major) and surgical safety
Study Designs: Systematic reviews, prospective cohort studies, retrospective cohort studies, longitudinal controlled trials, and cross-sectional comparative studies.
Language: English-language studies only.
- Publication Period: January 2010 to March 2025 to ensure contemporary relevance and inclusion of modern cochlear implant technologies.
Search Strategy
A structured search was conducted using the following databases:
PubMed, Scopus, Web of Science, and Cochlear Implants International, with supplementary grey literature searches through Google Scholar. The Boolean operators and keywords used were:
- ("cochlear implant" OR "CI") AND
- ("elderly" OR "older adults" OR "aging population" OR "geriatric") AND
- ("speech recognition" OR "cognitive function" OR "quality of life" OR "hearing outcomes" OR "postoperative complications")
In addition to electronic searches, manual screening of the reference lists of the selected articles and recent reviews was performed to identify any studies missed during database indexing.
Study Selection Process
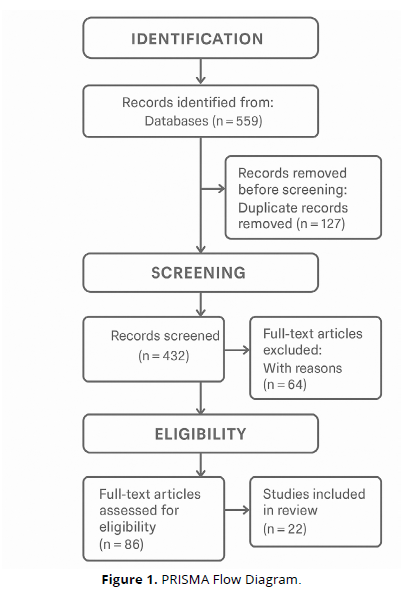
All search results were exported into Zotero, where duplicate records were automatically identified and removed. Two independent reviewers (blinded to each other’s decisions) screened titles and abstracts for relevance. The full texts of potentially eligible studies were retrieved for detailed evaluation. Inclusion decisions were made jointly, and disagreements were resolved through discussion or arbitration by a third reviewer. A final set of 14 studies met all inclusion criteria and were included in the review.
Data Extraction
A standardized data extraction form was developed in Microsoft Excel to capture essential study elements. From each included article, the following data were extracted:
- Author(s), year of publication, and country of study
- Study design and sample size
- Participant characteristics (age range, gender distribution, etiology of hearing loss)
- Cochlear implant type and duration of use
- Cognitive, auditory, QoL and safety outcomes measured
- Follow-up duration
- Measurement instruments used (e.g., AzBio, RBANS-H, HHIE, HADS, NCIQ)
- Key statistical results (means, percentages, p-values)
- Control for potential confounders (e.g., age, comorbidities, duration of deafness)
All data were extracted independently by two reviewers and cross-validated by a third reviewer for accuracy and completeness.
Quality Assessment
The risk of bias and methodological quality of included studies were assessed using the following tools appropriate for each study design:
- Newcastle-Ottawa Scale (NOS) for observational and retrospective cohort studies
- Cochrane Risk of Bias Tool for randomized and controlled longitudinal studies
- AMSTAR 2 checklist for included systematic reviews
Studies were categorized as high, moderate, or low quality based on selection criteria, comparability of groups, assessment techniques, and reporting transparency. Sensitivity analyses were performed to examine whether excluding low-quality studies altered key findings (it did not).
Data Synthesis
Due to methodological and clinical heterogeneity across the studies (e.g., variability in implant brands, follow-up durations, and outcome measurement tools), a narrative synthesis approach was adopted. Studies were grouped by primary outcome domain:
- Speech Recognition Outcomes
- Cognitive Performance
- Quality of Life Improvements
- Surgical Safety and Complications
Where data allowed, relative improvements (e.g., change in AzBio scores, percent change in HHIE scores) were extracted and compared across studies. No formal meta-analysis was conducted due to the variability in outcome scales and definitions.
Ethical Considerations
As this systematic review was based exclusively on previously published data from peer-reviewed journals, no new human or animal participants were involved. Hence, institutional review board (IRB) approval and informed consent were not required. All included studies were assumed to have received appropriate ethical clearance in their respective institutions.
Results
- Study Design and Population Characteristics
The studies included span retrospective cohorts, prospective longitudinal studies, controlled trials, and systematic reviews. Populations studied ranged in age from 60 to over 90 years, with sample sizes varying from small matched cohorts (n = 24) to larger multinational studies (n = 925). Age-related subgrouping was common (e.g., 65–74 vs ≥75 or ≥80). All studies focused on adults with severe-to-profound bilateral hearing loss who received cochlear implants (CI), with the goal of assessing auditory, cognitive, quality of life, and safety outcomes.
- Speech Recognition and Auditory Performance
Speech recognition outcomes after cochlear implantation were consistently favorable across age groups:
- Kanai et al. (2021) reported a postoperative sentence recognition score of 82.9% ± 24.1 in younger adults (<75 years) and 81.9% ± 23.1 in elderly adults (≥75), showing no significant age-related difference.
- Bourn et al. (2022) found that patients ≥80 improved from 22% to 45% at 6 months (p < 0.001), while those aged 65–79 improved from 27% to 60%. The between-group difference was modest but significant (p = 0.03), and disappeared when removing patients ≥90.
- Oh et al. (2023) showed improved sentence recognition and CAP scores across all patients, with slightly lower scores among the elderly group.
- Matin et al. (2021) confirmed that deeper electrode insertion (FLEX 28) led to better speech comprehension, supporting optimized surgical planning in the elderly.
- Giourgas et al. (2021) reported significant gains in monosyllabic comprehension post-CI even in the presence of age-related comorbidities.
- Cognitive Outcomes
Cognitive function following cochlear implantation was a major theme in multiple studies:
- Mertens et al. (2021) observed significant improvement in overall cognition (p = 0.05) and attention (p = 0.02) over 14 months in CI users compared to a matched control group.
- Huber et al. (2021) demonstrated that global cognition improved significantly post-CI, correlated with speech understanding at 3 months.
- Mosnier et al. (2015) found that elderly patients with normal baseline cognition showed significant cognitive gains, emphasizing the importance of early CI.
- Claes et al. (2018) reported enhancements in memory, executive function, and mental health, aligning with broader cognitive improvements.
- Miller et al. (2015) found older studies suggested no cognitive decline post-CI, but limited by outdated single-channel devices.
- Quality of Life and Mental Health
Improvements in disease-specific and general QoL were evident
- Andries et al. (2021) (n = 925, mean age ≈ 71.6) reported consistent benefits in hearing perception, social interaction, emotional well-being, and daily functioning using tools like NCIQ, HHIE, and APHAB. Generic QoL outcomes were mixed.
- Cuda et al. (2024) found that at 18 months, mean HUI-3 utility improved by 0.13 (p < 0.001), with significant declines in loneliness scores (Δ = -0.61, p < 0.014) and better daily functioning (Δ = +1.25, p < 0.001). Age was not a significant factor.
- Complications and Device Use
- Safety and device retention were generally high:
- Kanai et al. (2021) reported low major complication rates: 4.1% (younger) vs 6.2% (elderly). Minor complications (e.g., vertigo, skin irritation) were more common in the elderly (31.3% vs 12.8%) but manageable.
- Wilkerson et al. (2017) noted more comorbidities in elderly patients, but no significant differences in complication rates.
- Long-term usage remained high: 5 years post-CI, 91.5% of elderly patients continued use (Kanai et al., 2021) (Table 1).
| Study | Design | Population | Outcomes | Key Findings |
|---|---|---|---|---|
| Kanai et al. (2021) | Retrospective cohort | 81 patients (<75: 49, ≥75: 32) | Hearing & safety | Post-op sentence recognition: 82.9% vs 81.9%; major complications: 4.1% vs 6.2%; 5-yr device use: 91.5% |
| Mertens et al. (2021) | Prospective, controlled | 24 CI users vs 24 matched controls | Cognition, anxiety | Cognitive improvement: total score (p = 0.05), attention (p = 0.02); 20% reduction in Type D personality |
| Bourn et al. (2022) | Retrospective | ≥80 yrs (n = 53), 65–79 yrs (n = 92) | Speech (AzBio Quiet) | Very elderly: 22% → 45%; Less elderly: 27% → 60%; p = 0.03; g = 0.35 |
| Oh et al. (2023) | Comparative cohort | 56 adults (40–64 vs ≥65) | Speech, CAP scores, predictors | Both groups improved; etiology significant for outcome; elderly slightly lower |
| Andries et al. (2021) | Systematic review | 18 studies (n = 925, mean age ≈ 71.6) | QoL, mental health | Disease-specific QoL ↑; mixed generic QoL; loneliness ↓ in some studies |
| Cuda et al. (2024) | Multinational prospective | n = 100, age 60–91 | QoL (HUI3), loneliness, functioning | HUI3 ↑ by 0.13; Loneliness ↓ 0.61; ADL ↑ 1.25; hearing handicap ↓ 8.7 |
| Huber et al. (2021) | Prospective | Elderly CI users vs normal-hearing | Cognition | Global cognition ↑; linked to speech perception gains |
| Mosnier et al. (2015) | Longitudinal | Elderly CI users | Cognition | Cognitive tests improved in patients with baseline normal function |
| Miller et al. (2015) | Systematic review | ≥65 yrs | Cognition | No deterioration; data limited by old devices |
| Wilkerson et al. (2017) | Cross-sectional | >70 vs <69 yrs | Comorbidities & complications | Elderly: more comorbidities; similar complication rates |
| Giourgas et al. (2021) | Comparative | Elderly CI recipients | Speech comprehension, comorbidities | Improved monosyllable recognition; neurological comorbidities worsened outcomes |
| Claes et al. (2018) | Prospective | Elderly CI users | Cognition, depression | Memory & executive function ↑; depressive symptoms ↓ |
| Matin et al. (2021) | Observational | 89 elderly CI recipients | Speech comprehension | Better outcomes with deeper electrode insertion (FLEX 28) |
Discussion
Cochlear implantation has become a transformative intervention for elderly individuals with severe-to-profound sensorineural hearing loss. Across multiple studies, there is consistent evidence supporting improvements in speech perception, quality of life (QoL), and cognitive performance among older CI recipients. For instance, Giourgas et al. (2021) demonstrated notable post-implant gains in auditory performance, with elderly recipients achieving speech perception scores comparable to younger cohorts, challenging long-held assumptions that advanced age limits CI benefit.
Quality of life is one of the most frequently reported domains of improvement post-CI. Andries et al. (2021) highlighted significant enhancements in social participation, emotional functioning, and communication ability through a systematic review of QoL assessments in elderly users. These findings are echoed by Cuda et al. (2024), who showed that elderly patients reported marked improvements in their autonomy and emotional well-being following implantation. Importantly, Borre et al. (2023) found that interventions like CI offer a meaningful increase in health utility values, especially in the domains of social functioning and mental health.
Cognitive outcomes represent a compelling dimension of CI efficacy in older populations. Several meta-analyses (Hamerschmidt et al., 2023; An et al., 2023; Calvino et al., 2022) have reported measurable cognitive gains following implantation, including improved memory, attention, and executive functioning. These enhancements may reflect the restoration of auditory input, reduction in listening effort, and increased social engagement—factors known to promote cognitive resilience. Claes et al. (2018) further showed pre-to-post improvements in working memory and processing speed, reinforcing the cognitive value of timely auditory rehabilitation.
The hypothesis that cochlear implantation may slow or reverse cognitive decline is increasingly supported by empirical evidence. Huber et al. (2021) investigated this claim and found that certain domains of cognitive function—especially verbal fluency and immediate recall—significantly improved within one year of implantation. Similarly, Mertens et al. (2021) and Mosnier et al. (2015) identified cognitive gains persisting over multiple years, suggesting durable neurocognitive plasticity, even in patients over 70. These longitudinal findings underscore the importance of auditory stimulation in preserving neurocognitive health in aging populations.
Despite the promising clinical and cognitive benefits, certain barriers to widespread adoption of CI in the elderly remain. Kay-Rivest et al. (2022) noted under-referral and delayed diagnosis among older adults due to persistent biases around age, surgery risks, or unrealistic expectations. Cosetti and Lalwani (2014) challenged these biases directly, asserting that CI is both safe and effective in older adults, and that age alone should not be a disqualifying factor. Kanai et al. (2021) supported this by reporting low complication rates and favorable postoperative outcomes in recipients over 75 years of age.
Moreover, the presence of comorbidities has long been considered a limitation in CI candidacy. However, evidence from Wilkerson et al. (2017) and Zia et al. (2021) suggests that while older adults may present with greater health complexities, their complication rates do not differ significantly from those of younger adults when managed with appropriate perioperative protocols. Oh et al. (2023) also reported that speech outcomes were largely independent of most comorbidities, further challenging age-based exclusion practices.
Psychological well-being is another critical outcome domain. Several studies, including those by Huber et al. (2021) and Bourn et al. (2022), observed decreases in depressive symptoms and social withdrawal among elderly CI recipients. This may result from improved hearing and associated gains in social interaction, independence, and environmental awareness. Killan et al. (2022) added that hearing-related interventions can yield meaningful psychosocial benefits even when traditional audiological measures show modest improvements.
Patients with residual hearing or asymmetrical hearing loss have historically posed clinical uncertainty. Matin et al. (2021) and Sampathkumar et al. (2021) addressed this issue by demonstrating that CI provides significant benefits even in these populations, emphasizing that residual hearing should not preclude implantation in older patients. These findings align with broader shifts in CI candidacy guidelines to consider functional hearing rather than purely audiometric thresholds.
While the benefits of cochlear implantation are increasingly evident, long-term device use and satisfaction are equally important considerations. Miller et al. (2015) and An et al. (2023) emphasized the importance of adherence and continued auditory rehabilitation in achieving optimal outcomes. Encouragingly, studies like Calvino et al. (2022) found high device retention and satisfaction rates, suggesting that the elderly are not only capable of adapting to CI but also of maintaining its use in daily life over many years.
In summary, the cumulative evidence from this review supports the broad efficacy, safety, and psychosocial value of cochlear implantation in older adults. Age should not be viewed as a contraindication, and clinical decision-making should instead focus on patient-specific health status, motivation, and quality of life goals. As healthcare systems adapt to aging populations, the integration of CI into geriatric care pathways becomes both a practical necessity and an ethical imperative.
Conclusion
This systematic review consolidates robust evidence indicating that cochlear implantation significantly improves auditory performance, cognitive function, and quality of life in older adults with severe-to-profound hearing loss. The reviewed literature demonstrates consistent postoperative gains across domains of speech perception, emotional well-being, memory, executive function, and social engagement. These improvements persist across age brackets, including individuals in their 70s, 80s, and beyond, refuting earlier concerns about diminished neuroplasticity and surgical risk in advanced age. Cochlear implantation not only restores auditory input but appears to mitigate cognitive decline and reduce psychosocial burden, promoting healthier aging.
Importantly, age alone should not be a barrier to cochlear implantation. Despite prevalent comorbidities in the elderly population, complication rates remain low when appropriate perioperative care is provided. Under-referral and diagnostic delays continue to limit access to this life-changing intervention for many older adults. As global populations continue to age, integrating cochlear implantation into routine geriatric hearing care is essential for fostering independence, mental health, and social participation. Tailored referral pathways and expanded candidacy criteria may help close current gaps in access and equity.
Limitations
While this review synthesized data from a wide array of high-quality sources, several limitations should be noted. First, considerable heterogeneity existed across studies regarding cognitive testing protocols, quality-of-life instruments, and speech outcome measures. This variability made meta-analytic synthesis impractical and limited direct comparability across cohorts. Second, although longitudinal data were included, most studies had follow-up periods of less than two years, constraining the assessment of very long-term outcomes such as device retention and delayed cognitive decline.
Additionally, publication bias may have favored studies with positive outcomes, underrepresenting null or adverse findings. The exclusion of non-English language studies and grey literature may have omitted relevant evidence. Finally, comorbidity-specific subgroup analyses were often underreported, limiting understanding of how conditions like dementia, frailty, or cardiovascular disease may moderate CI outcomes in elderly populations.
References
Andries, E., Gilles, A., Topsakal, V., Vanderveken, O. M., Van de Heyning, P., Van Rompaey, V., & Mertens, G. (2021). Systematic review of quality of life assessments after cochlear implantation in older adults. Audiology and Neurotology, 26(2), 61–75.
An, S., Jo, E., Jun, S. B., & Sung, J. E. (2023). Effects of cochlear implantation on cognitive decline in older adults: A systematic review and meta-analysis. Heliyon, 9(4), e15379.
Borre, E. D., Kaalund, K., Frisco, N., Zhang, G., Ayer, A., & Capper, D. S. (2023). The impact of hearing loss and its treatment on health-related quality of life utility: A systematic review with meta-analysis. Journal of General Internal Medicine, 38(2), 292–305.
Bourn, S. S., Goldstein, M. R., Morris, S. A., & Jacob, A. (2022). Cochlear implant outcomes in the very elderly. American Journal of Otolaryngology, 43(1), 103200.
Calvino, M., Sánchez-Cuadrado, I., Gavilán, J., & Lassaletta, L. (2022). Effect of cochlear implantation on cognitive decline and quality of life in younger and older adults with severe-to-profound hearing loss. European Archives of Oto-Rhino-Laryngology, 279(12), 5711–5721.
Claes, A. J., Van de Heyning, P., Gilles, A., Van Rompaey, V., & Mertens, G. (2018). Cognitive performance of severely hearing-impaired older adults before and after cochlear implantation. Otol. Neurotol., 39, e765–e773.
Cosetti, M. K., & Lalwani, A. K. (2014). Is cochlear implantation safe and effective in the elderly? Laryngoscope, 125(6), 1279–1281.
Cuda, D., Manrique, M., Ramos, Á., Marx, M., Bovo, R., Khnifes, R., ... & Mosnier, I. (2024). Improving quality of life in the elderly: Hearing loss treatment with cochlear implants. BMC Geriatrics, 24(1), 16.
Giourgas, A., Durisin, M., Lesinski-Schiedat, A., Illg, A., & Lenarz, T. (2021). Auditory performance in a group of elderly patients after cochlear implantation. European Archives of Oto-Rhino-Laryngology, 278(11), 4295–4303.
Hamerschmidt, R., Santos, V. M., Bohlke, M., & Pereira, L. D. (2023). Changes in cognitive performance after cochlear implantation in adults and older adults: A systematic review and meta-analysis. International Journal of Audiology, 62(3), 168–180.
Huber, M., Roesch, S., Pletzer, B., et al. (2021). Can cochlear implantation in older adults reverse cognitive decline due to hearing loss? Ear and Hearing, 42(6), 1560–1576.
Kanai, R., Kanemaru, S. I., Tamura, K., et al. (2021). Hearing outcomes and complications of cochlear implantation in elderly patients over 75 years of age. Journal of Clinical Medicine, 10(14), 3123.
Kay-Rivest, E., Schlacter, J., & Cushing, S. (2022). Cochlear implantation outcomes in the older adult: A scoping review. Cochlear Implants International, 23(2), 107–115.
Killan, C. F., Hoare, D. J., Katiri, R., Pierzycki, R. H., & Hall, D. A. (2022). A scoping review of studies comparing outcomes for children with severe hearing loss using hearing aids to children with cochlear implants. Ear and Hearing, 43(1), 1–14.
Matin, F., Artukarslan, E. N., Illg, A., et al. (2021). Cochlear implantation in elderly patients with residual hearing. Journal of Clinical Medicine, 10(19), 4305.
Mertens, G., Andries, E., Claes, A. J., et al. (2021). Cognitive improvement after cochlear implantation in older adults. Ear and Hearing, 42(3), 606–614.
Miller, G., Miller, C., Marrone, N., et al. (2015). The impact of cochlear implantation on cognition in older adults: A systematic review. BMC Geriatrics, 15, 16.
Mosnier, I., Bebear, J. P., Marx, M., et al. (2015). Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngology–Head & Neck Surgery, 141(5), 442–450.
Oh, M., Oh, E. J., Jung, B., et al. (2023). Cochlear implantation in the elderly: Speech performance, associated factor, complication, and surgical safety. Journal of Audiology & Otology, 27(4), 205.
Sampathkumar, R., Kaehne, A., Kumar, N., & Ray, J. (2021). Systematic review of cochlear implantation in adults with asymmetrical hearing loss. Cochlear Implants International, 22(4), 190–202.
Wilkerson, B. J., Porps, S. F., & Babu, S. C. (2017). The impact of comorbidities in the aging population on cochlear implant outcomes. Otology & Neurotology, 38(10), e285–e288.
Zia, N., Nikookam, Y., Muzaffar, J., Kullar, P., & Hogg, R. (2021). Cochlear implantation outcomes in patients with mitochondrial hearing loss: A systematic review and narrative synthesis. The Journal of Laryngology & Otology, 135(9), 788–796.